Presented By: Brian Warnecke, DO – UC-Irvine Hematology Oncology Fellow, PGY-5
Date: August 12, 2023
The presentation titled “Multi-omic Characterization of RCC1 Expression and its Association with Molecular Alterations, Immune Phenotypes, and Cancer Outcomes,” conducted by Brian Warnecke, D.O., a UC-Irvine Hematology Oncology Fellow in his PGY-5, unveils a comprehensive investigation into the multifaceted role of Regulator of Chromosome Condensation 1 (RCC1) in cancer development and progression. Collaboratively led by principal investigators Dr. Mohammed Al Hallak (Karmanos), Dr. Asfar Azmi (Karmanos), and Dr. Misako Nagasaka (UC Irvine), this research endeavor delves into the intricate interplay between RCC1 expression, molecular alterations, immune characteristics, and patient outcomes.
The objectives of this study are fourfold: firstly, to elucidate the contribution of RCC1 in driving cancer development; secondly, to discern the correlation between RCC1 expression and key immune factors like PD-L1 expression, tumor mutational burden (TMB), and immune cell count within the tumor microenvironment; thirdly, to scrutinize the co-alterations occurring in RCC1-overexpressing solid tumors; and lastly, to ascertain the connection between RCC1 overexpression and patient outcomes.
The presentation begins by introducing RCC1 as the Regulator of Chromosome Condensation 1, responsible for governing nuclear import and export processes. It references the work of Azmi et al. in Nature Review Clinical Oncology (2021) to establish the importance of RCC1 in cellular functions.
The study methodology incorporates a wide range of cancer types, encompassing NSCLC, SCLC, pancreatic, colorectal, and gastric cancers. The analysis involves utilizing DNA (592-gene or WES) and RNA (WTS) data to quantify RCC1 expression. The presentation highlights RCC1 expression quartiles (with Q1 indicating low expression and Q4 representing high expression), TMB, PD-L1 tumor proportion score (TPS), and next-generation sequencing (NGS) techniques. Moreover, Kaplan Meier survival analysis is employed to evaluate overall survival (OS) outcomes.
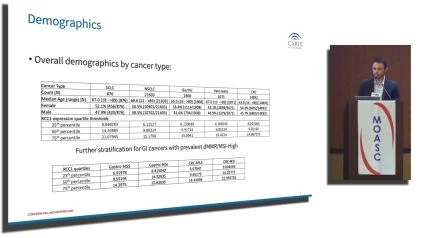
Demographic insights reveal a detailed breakdown of each cancer type, accounting for counts, median ages, and gender distribution among patients.
The impact of RCC1 expression on immune-related biomarkers is explored, unveiling trends of TMB-High rates progressively increasing from low (Q1) to high (Q4) RCC1 expression. Similarly, MSI-High rates show comparable patterns in gastric and colorectal cancers. However, PDL1+ rates demonstrate varied trends across cancer types, indicating the nuanced role of RCC1 in influencing immune phenotypes.
The presentation underscores co-alterations associated with RCC1 expression across distinct cancer types. For instance, in SCLC and NSCLC, mutation rates of TP53, RB1, and EGFR increase progressively with RCC1 expression. In pancreatic cancer, TP53 mutations and MYC amplifications correlate with higher RCC1 expression, while in CRC-MSS, various mutations and amplifications, such as TP53, APC, FBXW7, PIK3CA, MYC, SETD2, POLE, and MSH2, exhibit progressive increments with RCC1 expression.
The study’s clinical outcomes analysis reveals significant findings. High RCC1 expression is associated with worse overall survival in NSCLC, pancreatic, and CRC. However, this association is not observed in SCLC or gastric cancer. Interestingly, the prognostic impact varies based on chemotherapy or immunotherapy treatment, further emphasizing the complex relationship between RCC1 expression and patient outcomes.
In conclusion, this research signifies the potential central role of RCC1 in cancer resistance, implying its suitability as a target for novel targeted drug development. The comprehensive investigation undertaken in this presentation sheds light on the intricate connection between RCC1 expression, molecular alterations, immune phenotypes, and clinical outcomes in various cancer types, providing a substantial foundation for future studies and therapeutic interventions.
Acknowledging the pivotal contributions of Caris Life Sciences, the presentation concludes by inviting questions and discussions, reflecting the collaborative nature of scientific advancement in oncology.