Akasha Dukkipati, MD at City of Hope
August 21, 2023
Akasha Dukkipati, MD at City of Hope, conducted an analysis of urothelial cancer studies spanning a decade, from 2012 to 2022. The aim was to scrutinize eligibility criteria due to concerns raised by a collaborative statement from ASCO and Friends of Cancer Research. The joint statement underscored the underrepresentation of certain clinical attributes resulting from overly stringent eligibility standards in clinical trials.
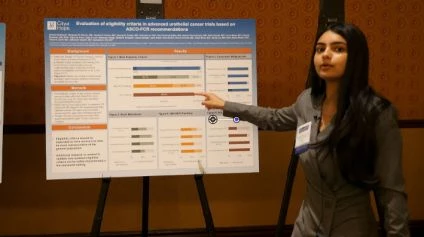
The physician’s team specifically focused on advanced urothelial cancer studies, examining four primary exclusion factors: concurrent malignancies, brain metastases, hepatitis B and C infections, and HIV. Their findings revealed that HIV positivity and hepatitis B and C positivity were associated with the most rigorous exclusion criteria.
For instances of HIV positivity, nearly 90% of trials led to exclusion. Unlike concurrent malignancies that sometimes featured conditional inclusion, HIV positivity lacked such flexibility. Within the HIV-positive cohort, both combination therapy and immunotherapy were subject to strict exclusion from these trials. This poses a dilemma as patients presenting with urothelial cancer along with HIV or hepatitis B/C infections lack comprehensive data on the most effective cancer therapies due to this data limitation.
The suggested course of action involves expanding the eligibility criteria to better represent the broader population, thus enabling comprehensive treatment strategies for these patients. The physician emphasized the graphical breakdown of findings, highlighting that HIV positivity demonstrated a high exclusion rate of 90%. Graphically, the darker bars symbolized complete exclusion, while the lighter hues represented conditional inclusion, indicating a more flexible approach. Some studies even lacked reported criteria, underscoring the need for expanding available data.