The FDA has recently approved Kisqali (ribociclib) in combination with a non-steroidal aromatase inhibitor (NSAI) for the treatment of early-stage HR+/HER2- breast cancer in patients at high risk of recurrence (download slides below). This approval provides a new treatment option for patients, aiming to reduce the likelihood of cancer recurrence during the early stages of the disease.
Key Findings from the NATALEE Trial
The NATALEE trial was a randomized, open-label, multicenter phase 3 trial that included 5,101 adults with HR-positive, HER2-negative early breast cancer. Patients were selected based on lymph node involvement or tumor size and grade, ensuring a diverse population was studied. Participants were randomized to receive ribociclib (400 mg) with NSAI or NSAI alone, with goserelin added for premenopausal women and male patients as needed.
Improved iDFS Rate
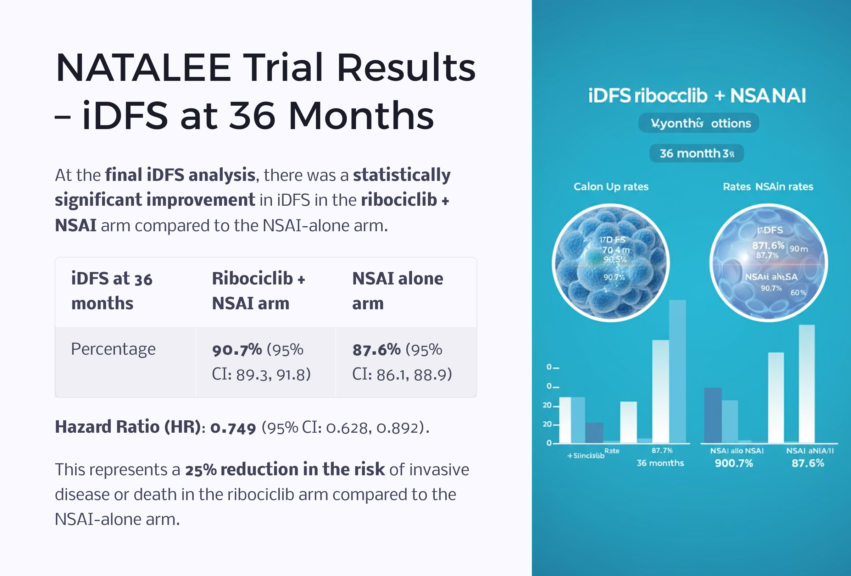
The primary efficacy outcome measured in the trial was invasive disease-free survival (iDFS). At 36 months, the iDFS rate was 90.7% in the ribociclib + NSAI arm compared to 87.6% in the NSAI-alone arm. This improvement, represented by a hazard ratio (HR) of 0.749, corresponds to a 25% reduction in the risk of invasive disease or death in the ribociclib arm, underscoring the effectiveness of CDK4/6 inhibition in preventing disease recurrence.
Safety Profile of Kisqali in the NATALEE Trial
The safety profile of Kisqali was consistent with what has been observed in metastatic settings. Common side effects included neutropenia (low white blood cell count), elevated liver enzymes, and fatigue. Patients undergoing treatment with ribociclib will require regular monitoring of blood counts and liver function to manage these potential risks effectively.
Conclusion
The FDA approval of Kisqali for early-stage high-risk HR+/HER2- breast cancer represents a major advancement in treatment, offering a new standard of care for preventing recurrence. Backed by the robust data from the NATALEE trial, ribociclib in combination with an aromatase inhibitor provides a promising option for high-risk patients. Continued monitoring of long-term survival outcomes will provide further insights into the full benefits of this therapy.
External Links and Sources:
FDA approves Kisqali with an aromatase inhibitor and Kisqali Femara Co-Pack for early high-risk breast cancer: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-kisqali-aromatase-inhibitor-and-kisqali-femara-co-pack-early-high-risk-breast-cancer
FDA approves Novartis Kisqali® to reduce risk of recurrence in people with HR+/HER2- early breast cancer: https://www.novartis.com/us-en/news/media-releases/fda-approves-novartis-kisqali-reduce-risk-recurrence-people-hrher2-early-breast-cancer






OncologyTube Links:
