Polatuzumab Vedotin: How can the POLARIX trial help us comprehend DLBCL? Connor Johnson MD
By P. Connor Johnson, MD
My name is Connor Johnson, and I am the medical oncologist at the Center for Lymphoma in the Massachusetts General Hospital Cancer Center. It is my privilege today to discuss the POLARIX trial, which potentially changes the standard of care for the treatment of diffuse large B-cell lymphoma, the most common lymphoma. The key takeaways from this talk are to review the current standard of care for diffuse large B-cell lymphoma and to recognize the significance of the POLARIX Phase III, randomized, double-blind, placebo-controlled trial (on polatuzumab vedotin) in our understanding of the evolving treatment of diffuse large B-cell lymphoma.
The R-CHOP Standard of Care
What was the previous standard of care for upfront treatment of diffuse large B-cell lymphoma? The Goya trial, a large clinical trial, showed the outcomes with the standard of care R-CHOP. R-CHOP consists of rituximab, an monoclonal antibody to CD20, a protein expressed on the outside of diffuse large B-cell lymphoma, along with a combination of prednisone, an oral corticosteroid, and three chemotherapy drugs named cyclophosphamide, adriamycin, and vincristine. This combination regimen has been the standard for over two decades, and the progression-free survival curve shows a cure rate of about 67%, or two-thirds of patients. Several clinical trials have been conducted to try and improve upon this without success to date.
What Kind of Drug is Polatuzumab Vedotin?
Polatuzumab vedotin is a relatively new drug in our armamentarium for diffuse large B-cell lymphoma. It is an monoclonal antibody drug conjugate to the protein CD79b and is conjugated to MMAE, a chemotherapy drug. It binds to CD79b and is internalized into cells. The FDA has already approved this drug in combination with bendamustine, a chemotherapeutic, and rituximab, the previously mentioned CD20 antibody (monoclonal antibody), for the treatment of relapsed or refractory diffuse large B-cell lymphoma, but not in the frontline setting.
The POLARIX Phase III, randomized, double-blind, placebo-controlled Trial of Polatuzumab Vedotin
A natural question has emerged about where this drug might apply in terms of upfront treatment of diffuse large B-cell lymphoma. The POLARIX trial sought to answer this question. It is a randomized trial comparing six full cycles of standard chemotherapy with R-CHOP with some additional treatments with rituximab to a new regimen, which is R-CHOP minus the vincristine and instead substituting polatuzumab vedotin with the same treatment-standard regimen of six cycles with two additional rituximab doses. The primary endpoint of this study is progression-free survival, an important endpoint in diffuse large B-cell lymphoma. Patients had to all have untreated DLBCL with higher-risk IPI scores of 2 or higher and a good performance status to participate in the study. There was stratification based on risk scores, like the IPI that we mentioned before, and the overall tumor size of the disease. These are some key characteristics of the patients who enrolled in the study. The two arms were well-matched, and there were no significant differences in terms of the actual patient populations. I’ll just note that these are patients that had primarily advanced-stage disease, higher-risk IPI scores, and a significant fraction of them with bulky disease, defined as more than 7 and 1/2 centimeters in the longest diameter of the disease.
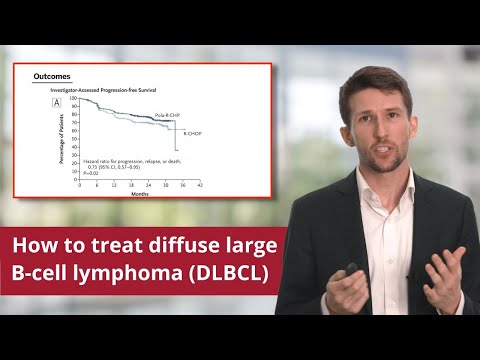
Here are the important outcomes from the POLARIX Phase III, randomized, double-blind clinical trial:
In Box A, we can see the progression-free survival curves that compare polatuzumab added to our R-CHP to standard R-CHOP. There is a 6.5% absolute difference in two-year progression-free survival between these two arms R-CHP and R-CHOP, in favor of polatuzumab.
In the other box, we can see the overall survival curves, which show no difference between the two arms. Importantly, toxicities were examined between the two regimens, and there were no significant differences, with similar rates of adverse effects, similar rates of grade 3 to 4 and grade 5 adverse effects, and similar rates of overall discontinuation of study drugs.
Final Thoughts About the Polatuzumab Vedotin: POLARIX Phase III, randomized, double-blind Clinical Trial?
So, what are the key takeaways from this new study in diffuse large B-cell lymphoma? This is really the first positive trial in upfront diffuse large B-cell lymphoma treatment in nearly two decades, so it is a remarkable addition to the overall knowledge base of diffuse large B-cell lymphoma. The overall benefit from progression-free survival is relatively modest, at 6.5% at 2 years as an absolute difference in favor of the polatuzumab vedotin arm. How this will emerge into the standard of care is currently being defined, and it remains to be seen. Thank you for watching. I hope this video has been educational.
10 Key Takeaways You Really Need to Know About the POLARIX Clinical Trial on Polatuzumab Vedotin
-
Polatuzumab Vedotin a monoclonal antibody is a targeted therapy that delivers a potent chemotherapy directly to cancer cells.
-
The trial involved patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), the most common type of non-Hodgkin lymphoma.
-
The trial was a phase II study, which means it was designed to evaluate the safety and efficacy of the treatment in a small group of patients.
-
The trial showed that the addition of Polatuzumab Vedotin to standard chemotherapy significantly improved overall response rates and progression-free survival compared to chemotherapy alone.
-
The trial also demonstrated that the treatment was generally well-tolerated, with manageable side effects.
-
Polatuzumab Vedotin received Breakthrough Therapy designation from the FDA based on the results of this trial.
-
The trial results led to the approval of Polatuzumab Vedotin in combination with chemotherapy for the treatment of relapsed or refractory DLBCL in the United States and Europe.
-
The trial results suggest that Polatuzumab Vedotin has the potential to improve outcomes for patients with relapsed or refractory DLBCL who have few treatment options.
-
The trial highlights the importance of developing targeted therapies that can deliver chemotherapy directly to cancer cells while minimizing damage to healthy tissue.
-
Further research is needed to explore the long-term benefits and optimal use of Polatuzumab Vedotin in DLBCL treatment, as well as its potential in other types of cancer.
Possible adverse effects of Polatuzumab Vedotin
Polatuzumab vedotin (monoclonal antibody) is a drug that has been tested in clinical trials for the treatment of Diffuse Large B-Cell Lymphoma (DLBCL), a type of cancer that affects the lymphatic system. The clinical trial of Polatuzumab vedotin, called the POLARIX trial, has shown promising results in the treatment of DLBCL, but like all clinical trials, it carries the risk of side effects.
Some of the common side effects that have been reported in the POLARIX trial include fatigue, nausea, diarrhea, fever, and decreased appetite. These side effects are usually mild to moderate in severity and can be managed with supportive care.
However, there are also some less common but more serious side effects that have been associated with Polatuzumab vedotin. These include:
-
Infections: Patients who receive Polatuzumab vedotin are at an increased risk of developing infections due to the drug’s effects on the immune system.
-
Infusion reactions: Some patients may experience allergic reactions during the infusion of Polatuzumab vedotin, which can range from mild to severe.
-
Neurological side effects: Polatuzumab vedotin has been associated with neurological side effects such as peripheral neuropathy, which is a condition that causes numbness, tingling, and pain in the hands and feet.
-
Liver toxicity: In rare cases, Polatuzumab vedotin has been associated with liver toxicity, which can cause liver damage and dysfunction.
It’s important to note that not all patients will experience these side effects, and the severity and duration of the side effects can vary from person to person. Patients should discuss any concerns about side effects with their healthcare professional before starting treatment with Polatuzumab vedotin.
Connor Johnson, MD – About The Author, Credentials, and Affiliations
P. Conner Johnson, MD, is a highly skilled and experienced healthcare professional specializing in radiation oncology. He currently serves as the Director of the Clinical Research and Outcomes Core in the Department of Radiation Oncology at Massachusetts General Hospital, and is also an Associate Professor of Radiation Oncology at Harvard Medical School.
Dr. Johnson received his medical degree from the University of Michigan Medical School, where he also completed his residency in radiation oncology. He went on to complete a fellowship in brachytherapy at the University of California, Los Angeles.
With over 20 years of experience in radiation oncology, Dr. Johnson is widely recognized for his expertise in treating patients with prostate cancer. He has authored numerous research publications on the topic, and is considered a leading expert in the use of advanced radiation therapy techniques for prostate cancer, including intensity-modulated radiation therapy (IMRT), image-guided radiation therapy (IGRT), and stereotactic body radiation therapy (SBRT).
Dr. Johnson is committed to providing compassionate, patient-centered care and is dedicated to helping his patients achieve the best possible outcomes. He is also passionate about research and has been involved in numerous clinical trials aimed at improving the effectiveness of radiation therapy for various types of cancer.
In addition to his clinical work and research, Dr. Johnson is actively involved in teaching and mentoring the next generation of radiation oncologists. He has served as a mentor to many medical students and residents and has been recognized for his outstanding contributions to medical education.
Overall, Dr. Patrick Johnson is a highly respected radiation oncologist who is committed to providing the highest quality care to his patients and advancing the field of radiation oncology through innovative research and education.

