In the evolving landscape of cancer care
In the evolving landscape of cancer care, the use of circulating tumor DNA (ctDNA), also known as liquid biopsies, is becoming increasingly significant in the management of colorectal cancer. This is true regardless of the stage at diagnosis. Recent discussions and studies have shed light on how ctDNA can guide treatment decisions, particularly in patients with stage II and III colorectal cancer.
For stage II colorectal cancer patients, the decision to administer adjuvant chemotherapy is nuanced and complex. It involves a detailed review of the pathology report and multiple risk factors. It’s important to communicate to patients that while chemotherapy does offer some benefit, there’s a risk of overtreatment. This is highlighted by studies like the dynamic study presented at Rasco and published in the New England Journal of Medicine. This study demonstrated that a ctDNA-directed approach could achieve similar outcomes with significantly fewer patients receiving chemotherapy. This is particularly relevant for those with low-risk pathology where traditional guidelines might suggest forgoing chemotherapy. However, if a patient’s ctDNA test comes back positive, it’s a strong indicator that they are not cured. Therefore, reconsideration of chemotherapy is prompted.
The distinction between pathologic high-risk features and a positive ctDNA test post-surgery is crucial. Pathologic features predict potential recurrence. Meanwhile, a positive ctDNA test directly informs both the patient and physician that there is residual disease. Given that ctDNA has a short half-life, its persistence post-operation signifies ongoing cancer activity. Studies show that untreated stage II patients with positive ctDNA typically recur within two years.
In clinical practice
In clinical practice, especially for stage II patients, a positive ctDNA result might influence the decision to opt for adjuvant chemotherapy. This tool is becoming an essential part of our decision-making arsenal. It provides personalized insights into patient care.
Regarding surveillance after adjuvant chemotherapy, the approach to ctDNA positivity is less clear due to the lack of definitive clinical trials. However, the analogy to rising CEA levels offers a guideline. Positive ctDNA might prompt further imaging, potentially including PET scans for functional imaging. MRI could be used for better detection of liver metastases, especially in high-risk scenarios like T4 tumors.
Excitingly, there are ongoing clinical trials recruiting patients with positive ctDNA who haven’t yet shown radiographic recurrence. These trials are exploring vaccine-based therapies and image-guided interventions. These trials, available in the US and globally, offer new avenues for treatment beyond standard care.
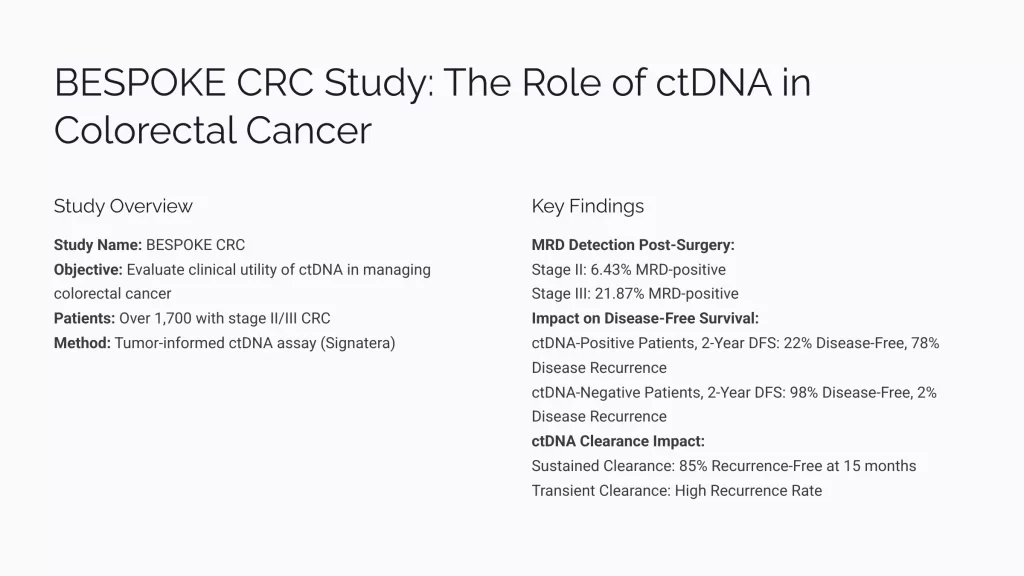
The data from studies like the bespoke study provide benchmarks for ctDNA positivity rates. This offers a clearer picture of prognosis and helps tailor discussions with patients about their recurrence risks. For instance, while ctDNA negativity post-treatment correlates with a high chance of being disease-free, positivity significantly increases the risk of recurrence.
When discussing ctDNA with patients
When discussing ctDNA with patients, it’s vital to set expectations before testing. For stage II and III patients, knowing the positivity rates can guide both treatment decisions and participation in clinical trials. While ctDNA negativity is promising, it’s not a definitive cure signal. Thus, chemotherapy might still be recommended based on other risk factors.
The integration of ctDNA testing into clinical practice continues to evolve. It provides not just prognostic but potentially predictive insights into colorectal cancer management. As we await more definitive data from randomized trials, ctDNA remains a provocative tool in our fight against colorectal cancer. It enhances our ability to personalize treatment and improve patient outcomes.
For more detailed information and to stay updated on the latest in colorectal cancer research, visit www.OncologyTube.com.
#ColorectalCancer #ctDNA #LiquidBiopsy #CancerTreatment #Oncology #ClinicalTrials #Chemotherapy #PrognosticTool #PersonalizedMedicine #PatientCare #MedicalResearch
Related Articles:
https://dailynews.ascopubs.org/do/bespoke-crc-findings-bolster-clinical-utility-ctdna-testing-crc