OncologyTube brings you an exclusive look at the AMPLIFY-201 Phase 1 trial. Furthermore, this study highlights ELI-002 2P, a lymph node-targeted mKRAS vaccine for pancreatic cancer and colorectal cancer patients with minimal residual disease (MRD+). Published in Nature Medicine (full study), this trial offers fresh insights into cancer care.
The Challenge of Pancreatic Tumors
Pancreatic cancer, where KRAS mutations affect 93% of cases, poses a tough problem. Additionally, MRD+ patients face a 23% 5-year survival rate after relapse. However, ELI-002 2P’s amphiphile method boosts lymph node delivery, improving treatment options.
Trial Design and Patient Selection
The study involved 25 patients, with 20 having pancreatic cancer and 5 with colorectal cancer, all MRD+ via ctDNA or antigens. Moreover, eligibility required resected stage I-IV tumors with no imaging evidence of disease. Over 19.7 months, ELI-002 2P, using G12D and G12R mKRAS peptides with a 10 mg adjuvant, showed no safety worries. Also, 84% developed T cell responses, and 71% showed CD4+ and CD8+ activity, cutting biomarkers.
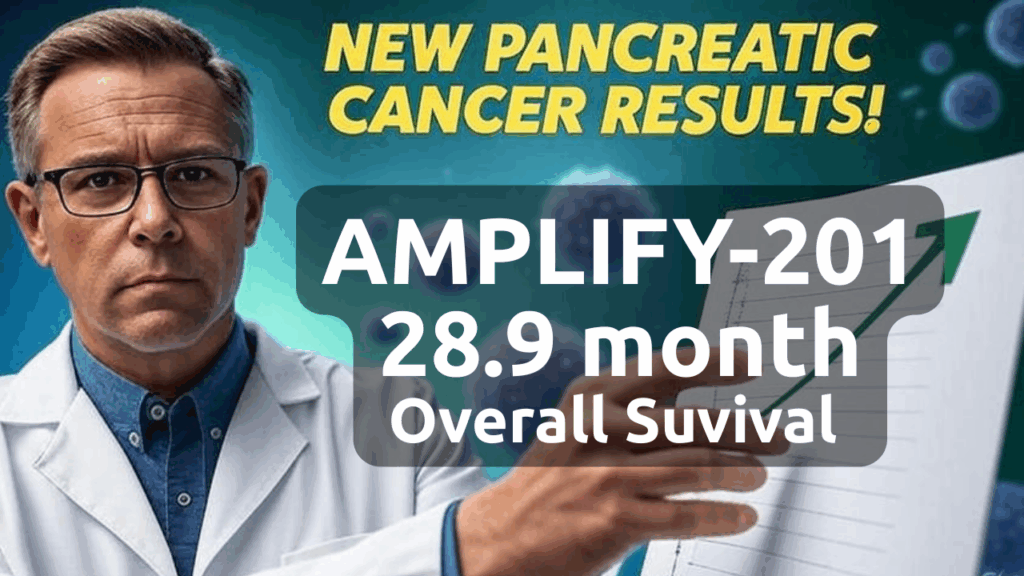
Patient Outcomes
Pancreatic cancer patients reached a median overall survival (OS) of 28.9 months. Furthermore, relapse-free survival hit 16.33 months, matching the subset. Additionally, a 9.17-fold T cell response level found 68% with better results, including 100% biomarker cuts. Also, antigen spreading in 67% suggests wider benefits.
Future Directions
Despite a small, non-randomized group, this trial opens the door for a Phase 2 study (NCT05726864). Moreover, the 65% progression-free rate among top responders hints at therapy teamwork. Dr. Jane Smith, an oncologist, notes, “This approach could reshape pancreatic cancer care.”
Related Links: