MRD: Inotuzumab, GMALL, Ponatinib & Blinatumomab ASH Updates in Acute Lymphoblastic Leukemia/Immunotherapy
By Van Huynh, MD
Today I spoke at MOASC (Medical Oncology Association of Southern California), and the focus was on acute lymphoblastic leukemia, or ALL, along with any new updates on immunotherapy in this population.
Did anything stand out to you about these clinical trials?
So I presented on three separate studies, and I think the general theme was that it acknowledged that the overall survival rate for adults with ALL (acute lymphoblastic leukemia) has historically been poor.
But there have been many. There have been several attempts to improve this overall survival, and one of the studies I presented was the GMALL study, which looked at a population of over 800 patients. It’s a German trial that included over 140 sites. And essentially what they did was to look at how patients were historically treated on conventional chemotherapy.
And then they also compared them with a second group called Group 2, where conventional chemotherapy was used, but with more of a pediatric backbone and using asparaginase mainly after induction and to assess efficacy and response to therapy. They used MRD (Minimal Residual Disease) or minimal residual disease to assess response and how well these patients did.
And so in that study, what they found was that when you look. The group one and group two, the group 2 fairs significantly better in terms of their overall survival, event-free survival. So the median age of this population was about 68. And what they found was that the patients who were younger fared much better in comparison to those who were 75 or older. And they also found that MRD (Minimal Residual Disease) was important, and that those who cleared their MRD (Minimal Residual Disease) at the end of the consolidation phase also had a much more significantly improved overall survival of about 80% at three years. And so I think this study, I think they concluded really that number one, using a pediatric patients based regimen could be beneficial for adults. Number two, if asparaginase, which is predominantly used in pediatric patients protocols, is used after induction, these patients do better.
I also forgot to mention that they saw less toxicity in this second group that used this type of regimen compared to the first group. But they also concluded that perhaps moving forward, what they can also think about is using immunotherapy in combination with conventional therapy to further improve overall survival for this de novo group of B-cell ALL (acute lymphoblastic leukemia), and also hopefully improve the toxicity that we see with chemo.
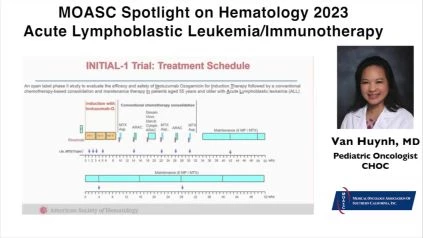
I also discussed at this meeting two other studies. One was using immunotherapy in combination with conventional chemotherapy. And that immunotherapy is called Inotuzumab, an anti-CD22 monoclonal antibody. The investigators, this is the GMALL INITIAL-1 trial looking at over 40 patients where they use Inotuzumab with a conventional chemotherapy. These patients were offered Inotuzumab three induction blocks MRD (Minimal Residual Disease) was used at the end of each block, and if the patients had molecular remission, they were then offered conventional chemotherapy thereafter, along with a maintenance therapy.
And what they found in this study is that these patients actually had very good overall survival. What they found at the end of this study was that actually all patients—some 40 patients had a complete remission, which was amazing. In addition to that, the overall survival at 1 and 2 years, respectively, was 91% and 81%. So I think that was pretty remarkable. They found that this agent was fairly well tolerated with minimal side effects, so a little bit of myelosuppression in combination with conventional chemotherapy so again, using this Inotuzumab with conventional therapy with De Novo ALL patients was promising.
I also reviewed another study where they looked at Ph-Positive newly diagnosed patients and combining Ponatinib with Blinatumomab. This study was also promising, Blinatumomab, as patients with Ph-Positive ALL can have a high risk incidence of relapse because of resistance to TKIs. And Ponatinib is a third generation drug that has the potential to overcome this resistance. And so, when they did the study and looked at these patients, they found that the combination of the two also showed great promise, and these patients also had an excellent overall survival. And so I think that there will be more to come from these studies as these patients will continue to with their therapy.
Watch and Share the Presentation with Slides Here: https://oncologytube.com/v/41750
Listen and Share the Audio Podcast Here:
What is MRD (Minimal Residual Disease) and next-generation sequencing or genomic sequencing?
After finishing a course of treatment, “complete remission” is one of the most wonderful things a patient may hear. On the basis of scans and laboratory testing (clinical trials or clinical studies), there is no evidence of malignancy, indicating that the treatment was effective.
However, there is an additional word that can be somewhat confusing for patients: minimum residual disease (MRD). When treating individuals with blood cancers such as leukemia, lymphoma, and multiple myeloma, this term is widely employed.
We currently have substantially more sensitive assays for evaluating MRD (minimum residual disease) These may involve next-generation sequencing (genomic sequencing), which allows us to examine samples of bone marrow for genetic mutations. Even if no disease residuals are visible under a microscope, the presence of mutations suggests their presence.
Flow cytometry allows us to evaluate the same samples for abnormal proteins on the cell surface. By estimating the amount of cells expressing abnormal proteins, we can gain a more precise understanding of the number of remaining cancer cells. We routinely use these innovative assays to evaluate whether a patient has MRD (minimal residual disease) after getting standard treatment.
There is a great deal to gain. These hematologic malignancies can adapt to therapy, which implies that the cancer we have before treatment and the cancer we have after treatment are not identical. By evaluating the minimal residual disease (MRD), we can have a better understanding of what remains after treatment.
This expedites multiple tasks. First, it allows us to modify our treatment by adding medications that target specific vulnerabilities in the cancer cells, including those that are highly effective at eradicating even residual cells, or by performing a stem cell transplant, which is capable of eliminating remaining cells.
What are your hopes for future research on this disease state?
So I think the future hope is one thing, is that a couple things is that, when you look at ALL (acute lymphoblastic leukemia), the population in pediatrics, they have an excellent survival. You’re looking at least 90 to 95%.
Even some sub groups low risk have 98.6, the adults don’t fare as well. In the past, you are seeing survival rates of less than 50%. We are seeing much better in the sixties now even. And then the study that I just mentioned to you, In the eighties. But the hope is that by using more targeted (therapy) immunotherapy therapy that targets CD 19, CD22, rather than saving it for the relapse population, perhaps moving it up front to the newly diagnosed and using Minimal Residual Disease (MRD) to stratify the patients that should and could use this and maybe even using Minimal Residual Disease (MRD) to tailor your therapy and risk stratify these patients. I think that potentially could, number one, improve survival, but decrease the toxicity and really move the pendulum so that these patients have a much better survival than what we’re seeing currently. And I think that this field is evolving greatly, and I’m hopeful that we can get the adults and the survival for ALL (acute lymphoblastic leukemia) to improve significantly.
Do you have anything to add about these studies for your peers?
I think what I’d like to add is I’d love to see more studies also that focus on, we see that the adults older adults have a less than optimal overall survival. But it would be wonderful to see also studies also focus in the adolescent young adults with ALL (acute lymphoblastic leukemia) using all these agents up front and also in the relapse setting because this is another population that doesn’t do as well with this disease.
Van Huynh, MD – About The Author, Credentials, and Affiliations
Van Huynh, MD, is a CHOC Children’s Oncology specialist. She holds board certifications in both pediatrics and pediatric hematology-oncology. Her clinical interests include acute lymphoblastic leukemia (ALL) in children, leukemia relapse, immunotherapy, and bone marrow transplantation.
Dr. Huynh attended the UC Irvine College of Medicine in California for medical school. She did her residency in pediatrics at Harbor-UCLA Medical Center, and then she did her fellowship in pediatric hematology-oncology at CHOC Children’s Hospital. She is the primary investigator (PI) for CHOC’s Therapeutic Advances in Childhood Leukemia and Lymphoma (TACL) Consortium, a multi-center collaboration that provides novel therapeutic choices for patients with relapsed or resistant leukemia and lymphoma. Dr. Huynh manages the CAR T cell program at CHOC Children’s and is the PI for the ZUMA-4 CAR T-cell Trial.
Reference
-
MD Anderson Cancer Center – What is minimal residual disease (MRD)? MD Anderson Cancer Center, July 15, 2020