The FDA has approved pembrolizumab (Keytruda) combined with platinum-based chemotherapy for the treatment of patients with unresectable, advanced, or metastatic malignant pleural mesothelioma (download slides below). This marks the first new treatment for mesothelioma in over 15 years, providing a new option for patients battling this aggressive disease.
Key Results from the KEYNOTE-483 Trial
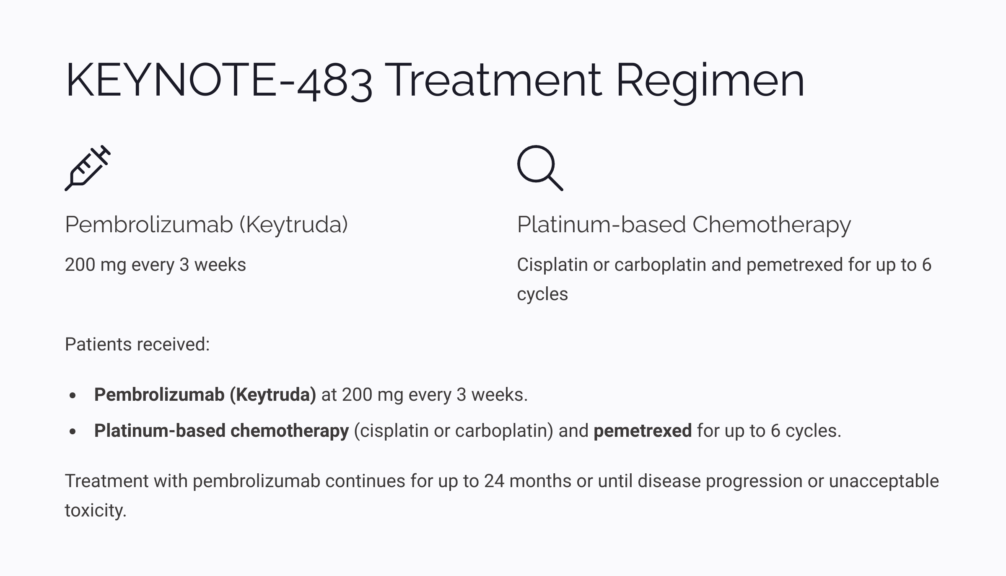
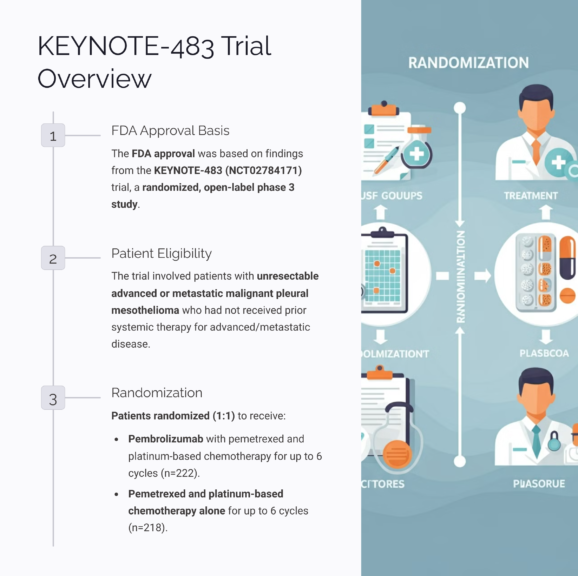
The approval was based on findings from the KEYNOTE-483 trial, a randomized, open-label, phase 3 study involving patients with unresectable, advanced, or metastatic malignant pleural mesothelioma who had not received prior systemic therapy. Participants were randomized to receive either pembrolizumab with pemetrexed and platinum-based chemotherapy for up to six cycles, or pemetrexed and platinum-based chemotherapy alone.
Efficacy Findings:
- Overall Survival (OS):
Median OS was 17.3 months (95% CI: 14.4, 21.3) for the pembrolizumab plus chemotherapy arm compared to 16.1 months (95% CI: 13.1, 18.2) in the chemotherapy alone arm, representing a 21% reduction in the risk of death (HR: 0.79). - Progression-Free Survival (PFS):
Median PFS was 7.1 months for both treatment arms, with a hazard ratio of 0.80 (95% CI: 0.65, 0.99), demonstrating a benefit in the pembrolizumab group. - Objective Response Rate (ORR):
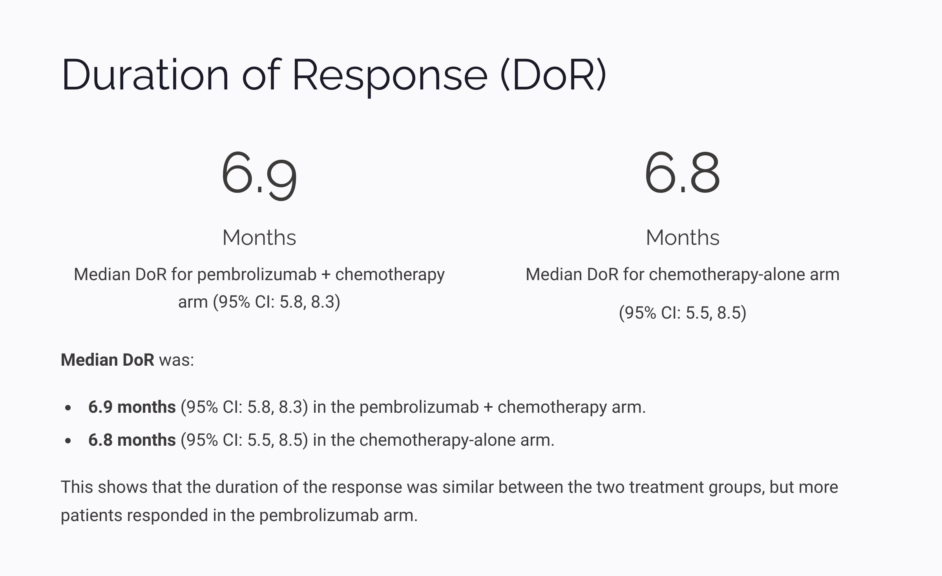
ORR was 52% in the pembrolizumab plus chemotherapy arm, compared to 29% in the chemotherapy-alone arm. - Duration of Response (DoR):
Median DoR was 6.9 months in the pembrolizumab arm and 6.8 months in the chemotherapy-alone arm.
Safety Profile
The safety profile of pembrolizumab in this study was consistent with findings in other cancers. Common adverse effects included:
- Fatigue
- Nausea
- Constipation
- Decreased appetite
Serious side effects included immune-mediated conditions such as pneumonitis, colitis, hepatitis, and nephritis, highlighting the need for regular patient monitoring.
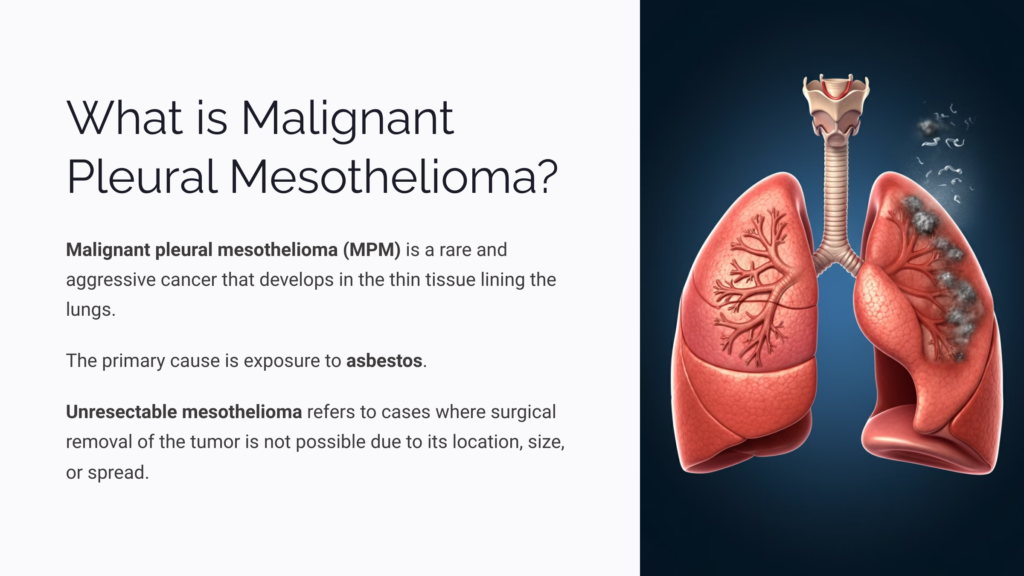
What is Malignant Pleural Mesothelioma?
Malignant pleural mesothelioma (MPM) is a rare and aggressive cancer that develops in the thin tissue lining the lungs, primarily caused by exposure to asbestos. In unresectable mesothelioma, surgical removal is not possible due to tumor location, size, or spread.
About Pembrolizumab: Pembrolizumab is an immune checkpoint inhibitor that targets PD-1, a protein found on T cells, enhancing the immune system’s ability to fight cancer cells. In combination with chemotherapy, pembrolizumab has demonstrated significant efficacy in improving survival rates for mesothelioma patients.
Conclusion:
This FDA approval marks a significant advancement in mesothelioma treatment, offering new hope to patients. Continued patient monitoring and further research will help solidify pembrolizumab’s role in treating advanced mesothelioma.






External Sources and Links:
FDA approves pembrolizumab with chemotherapy for unresectable advanced or metastatic malignant pleural mesothelioma: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pembrolizumab-chemotherapy-unresectable-advanced-or-metastatic-malignant-pleural
FDA Approves Merck’s KEYTRUDA® (pembrolizumab) Plus Pemetrexed and Platinum Chemotherapy as First-Line Treatment for Adult Patients With Unresectable Advanced or Metastatic Malignant Pleural Mesothelioma (MPM): https://www.merck.com/news/fda-approves-mercks-keytruda-pembrolizumab-plus-pemetrexed-and-platinum-chemotherapy-as-first-line-treatment-for-adult-patients-with-unresectable-advanced-or-metastatic-malignant-pleu/
OncologyTube Links:
