Date: February 8, 2025
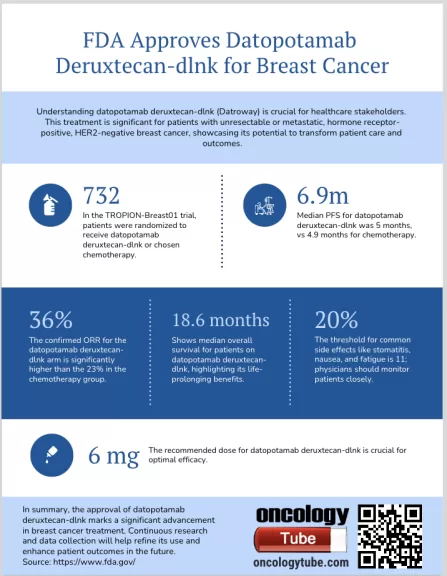
The U.S. Food and Drug Administration (FDA) has recently granted approval for datopotamab deruxtecan (DLNK), a new treatment option for adults with unresectable or metastatic hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative breast cancer. This marks a significant advancement in the fight against breast cancer, particularly for those who have progressed after prior endocrine-based therapies.
Key Points:
- Drug Name: Datopotamab deruxtecan (DLNK)
- Indication: Treatment for adults with unresectable or metastatic HR-positive, HER2-negative breast cancer who have received prior systemic therapy in the metastatic setting, or have progressed within 12 months of completing adjuvant therapy.
- Mechanism: Datopotamab deruxtecan is an antibody-drug conjugate that targets topoisomerase I inhibitor to cancer cells.
This approval provides hope and an additional therapeutic avenue for patients facing advanced stages of this type of breast cancer, potentially improving outcomes where previous treatments have failed.
For more detailed information, including clinical trial data, safety, and prescribing information, please visit the FDA’s official announcement.
Share your thoughts or experiences in the comments below. Let’s discuss how this approval might impact the breast cancer community!
This post encourages engagement by inviting comments, which could foster a community discussion around the new treatment’s implications, patient experiences, or questions about the drug. Remember to keep the conversation respectful and informative.
Related Articles:
