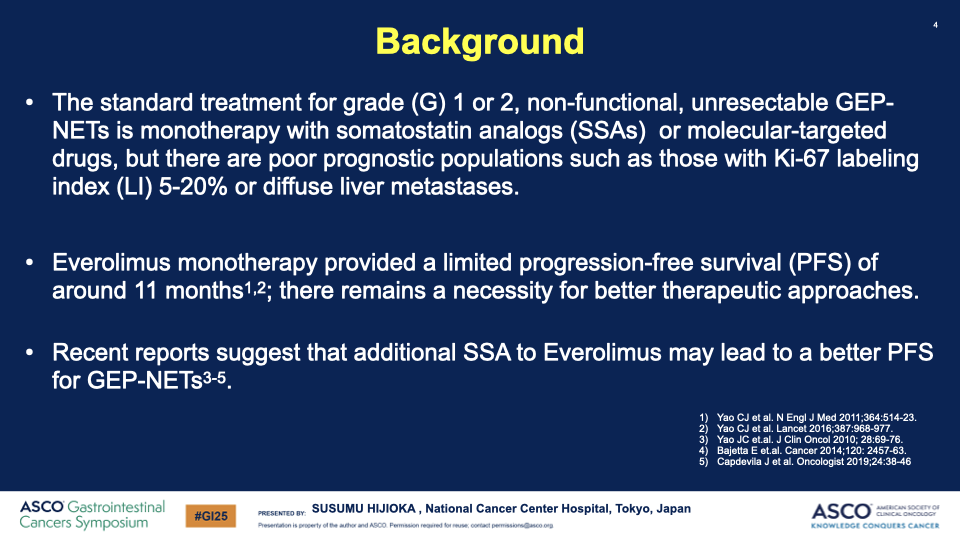
Background: The treatment landscape for well-differentiated gastroenteropancreatic neuroendocrine tumors (GEP-NETs) has been slow to evolve, with limited evidence supporting the addition of somatostatin analogs to molecular targeted therapies. STARTER-NET A recent phase III trial aimed to fill this gap by comparing the efficacy of everolimus (EVE) combined with lanreotide (LAN) against everolimus alone in patients with advanced GEP-NETs.
Trial Details:
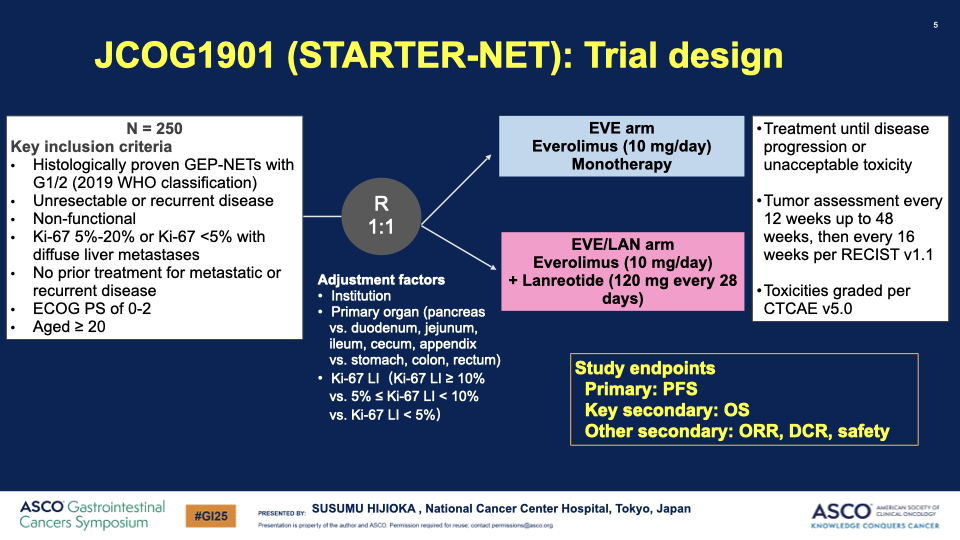
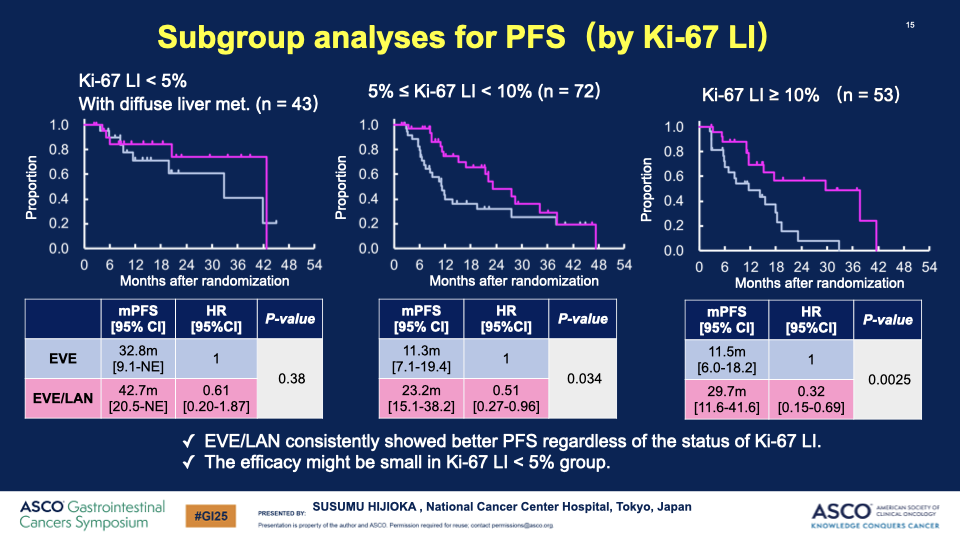
- Participants: Patients with nonfunctioning, grade 1 or 2 GEP-NETs with poor prognostic indicators, including a Ki-67 index of 5-20% or diffuse liver metastases, were enrolled.
- Treatment Arms: Patients were randomly assigned to receive either:
- Everolimus (10 mg/day) alone or
- Everolimus (10 mg/day) plus Lanreotide (120 mg every 28 days).
- Endpoints: The primary endpoint was progression-free survival (PFS), with secondary endpoints including overall survival (OS), objective response rate (ORR), disease control rate (DCR), and safety.
Key Findings:
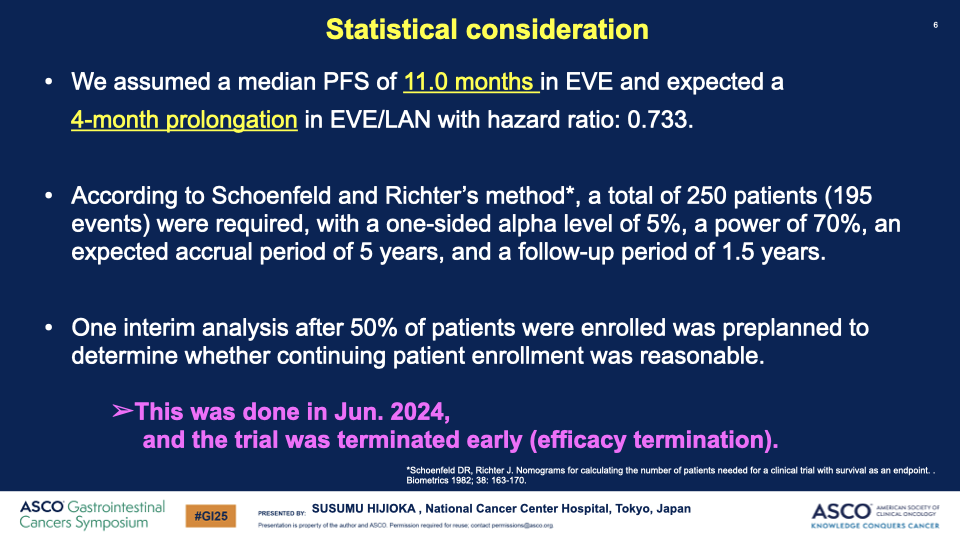
- Enrollment and Analysis: From April 2020 to June 2024, 178 patients were enrolled, with an interim analysis on 145 patients conducted in June 2024.
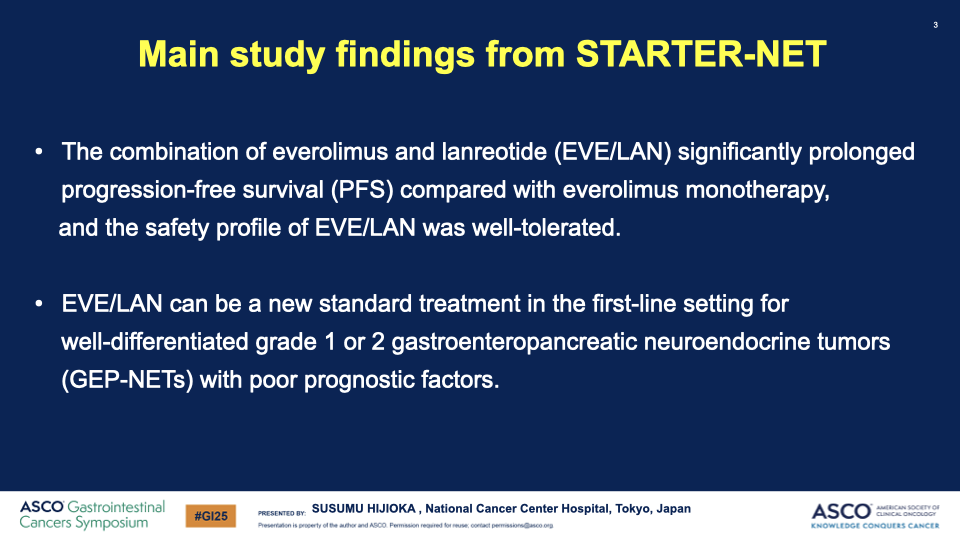
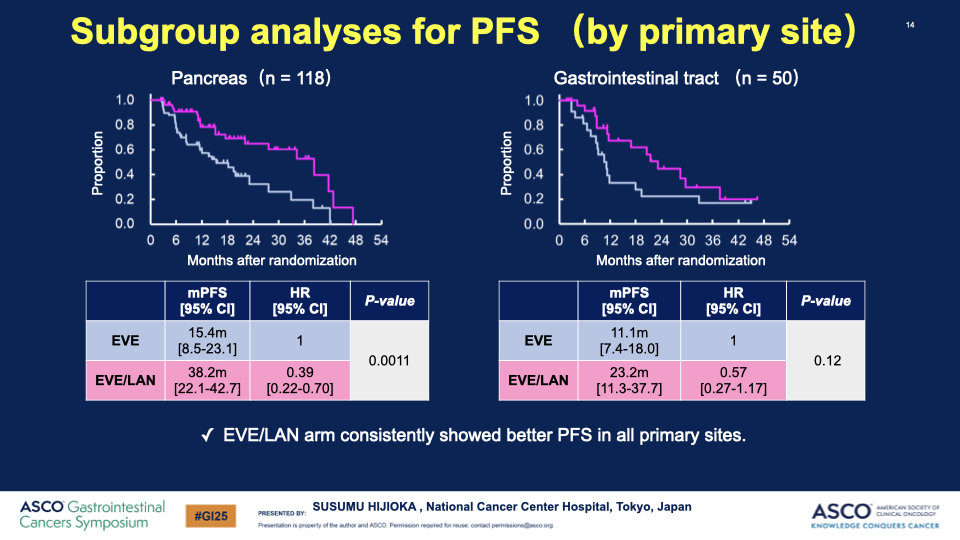
- PFS Results: The median PFS significantly improved from 11.5 months in the EVE group to 29.7 months in the EVE/LAN group, with a hazard ratio of 0.38, indicating a substantial benefit of the combination therapy.
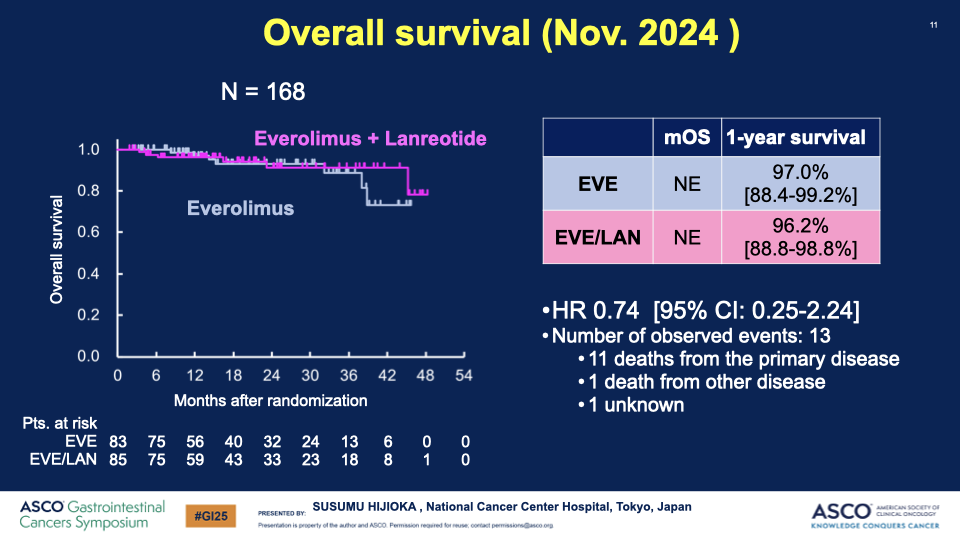
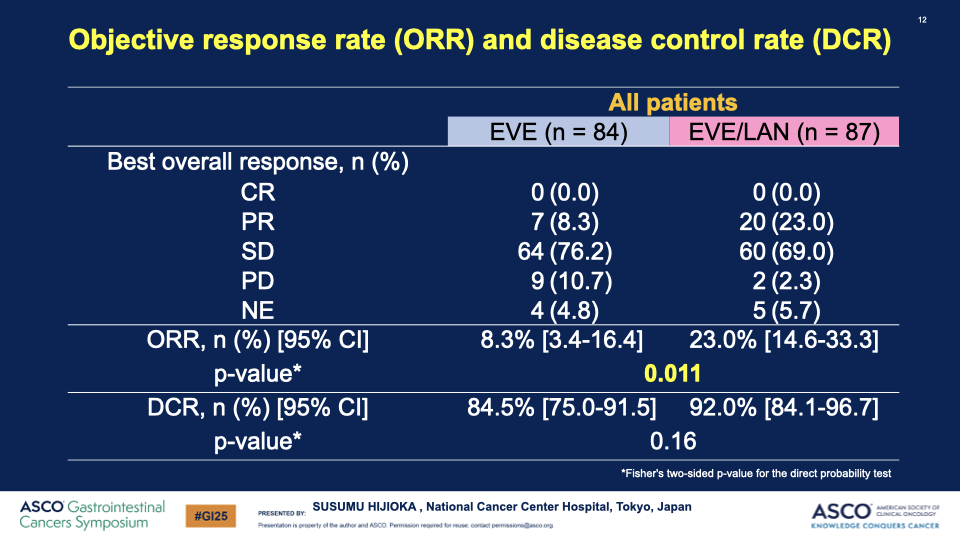
- OS and Response Rates: While the hazard ratio for OS was not significant (0.97), there was a notable increase in both ORR (8.7% to 26.8%) and DCR (87.0% to 91.5%) in the combination arm.
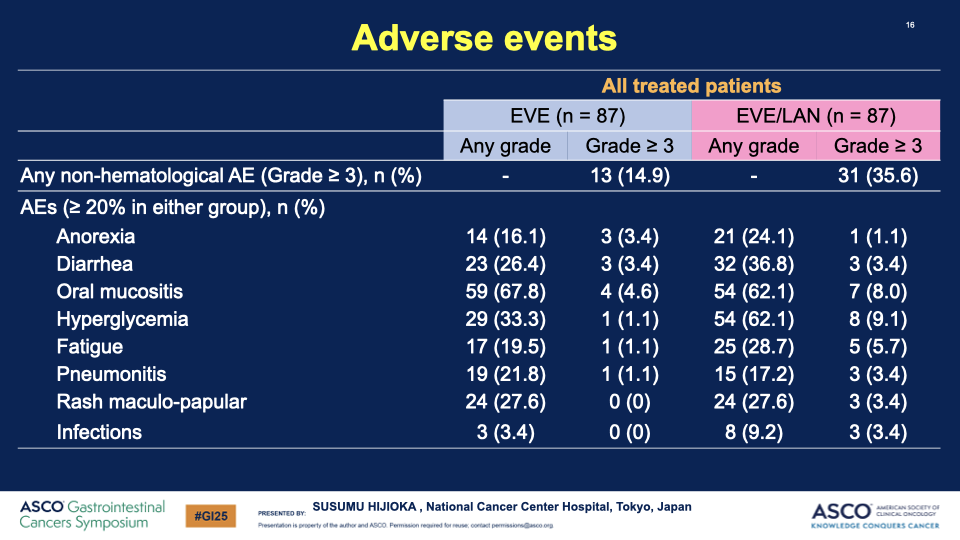
- Safety: The combination therapy showed increased frequency of both hematologic and non-hematologic toxicities, yet no treatment-related deaths were reported, suggesting a manageable safety profile.
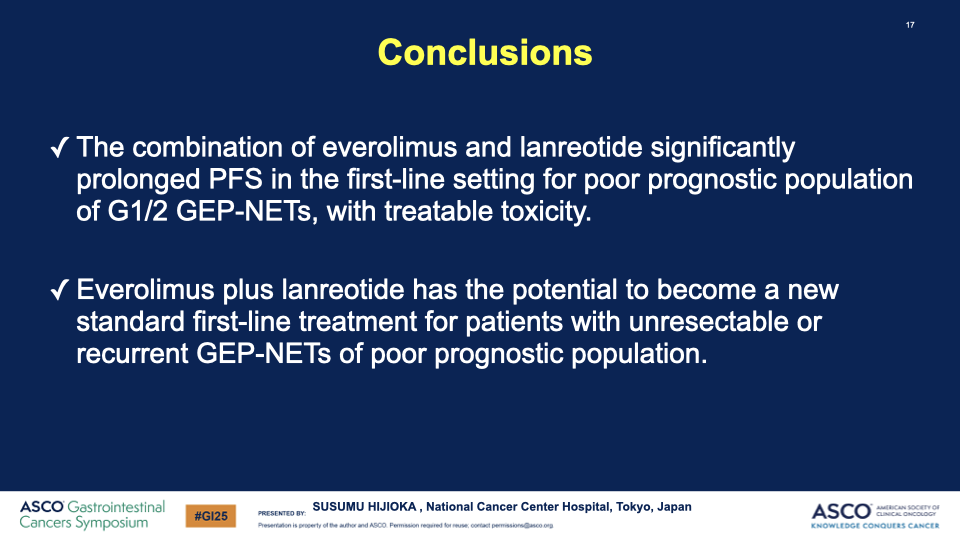
Conclusion: The combination of everolimus and lanreotide has shown a statistically significant increase in progression-free survival for patients with well-differentiated GEP-NETs with poor prognostic factors. This outcome led the Data and Safety Monitoring Committee to recommend early termination of the study due to clear efficacy benefits. The combination therapy could potentially set a new standard for first-line treatment in this patient group.
Impact: This trial’s results could influence treatment guidelines, offering hope for better outcomes in managing GEP-NETs with targeted therapies combined with somatostatin analogs. Further research will continue to explore long-term survival benefits and optimal management strategies for side effects.
Video Chapters:
Chapter 1: Introduction to Combination Therapy
00:00:00 Description: Overview of the synergistic effects of Everolimus Plus Lanreotide, focusing on good results in PFS. Chapter
2: Subgroup Analysis
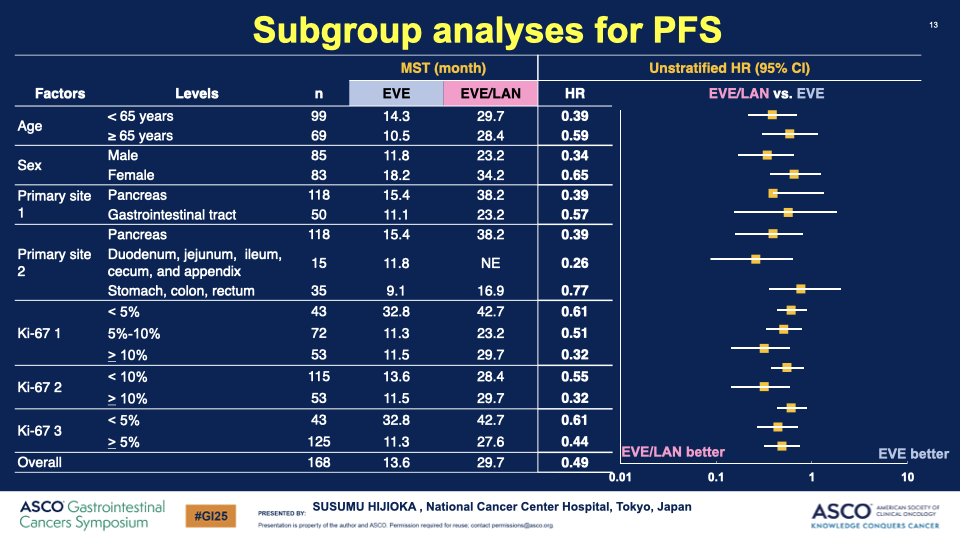
00:00:19 Description: Discussion on how specific subgroups, particularly those with a Ki-67 index over 10%, show stronger effects from the therapy.
Chapter 3: Side Effects and Management
00:00:34 Description: Explanation of hyperlyceia as a side effect and the importance of glucose monitoring and potential diabetic treatment.
Chapter 4: Challenges in Determining Efficacy
00:01:16 Description: Discussion on the difficulty of measuring efficacy via OS due to the long natural course of the disease, setting OS as a key secondary endpoint.
Chapter 5: Confirmation of Therapy Effects
00:01:48 Description: Confirmation of the hazard ratio consistency with PFS outcomes.
Chapter 6: Mechanisms of Therapy
00:02:00 Description: Explanation of how the therapy blocks molecular pathways, leading to higher response rates.
Chapter 7: Future Research Directions
00:02:21 Description: Considerations for future trials, focusing on higher Ki-67 groups and head-to-head comparisons with other treatments in NET G2 and G3 groups.
Stay Updated: For updates on this research or to learn more about the treatment of GEP-NETs, keep an eye on clinical trial registries and oncology news platforms.
Official Presentation Slides Courtesy Dr Susumu Hijioka, MD – Department of Hepatobiliary and Pancreatic Oncology, National Cancer Center (Japan):


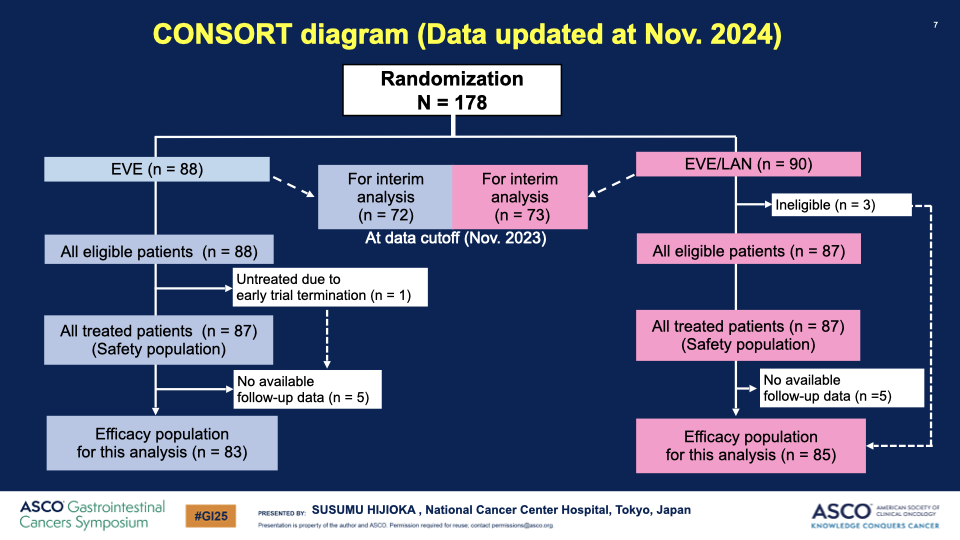
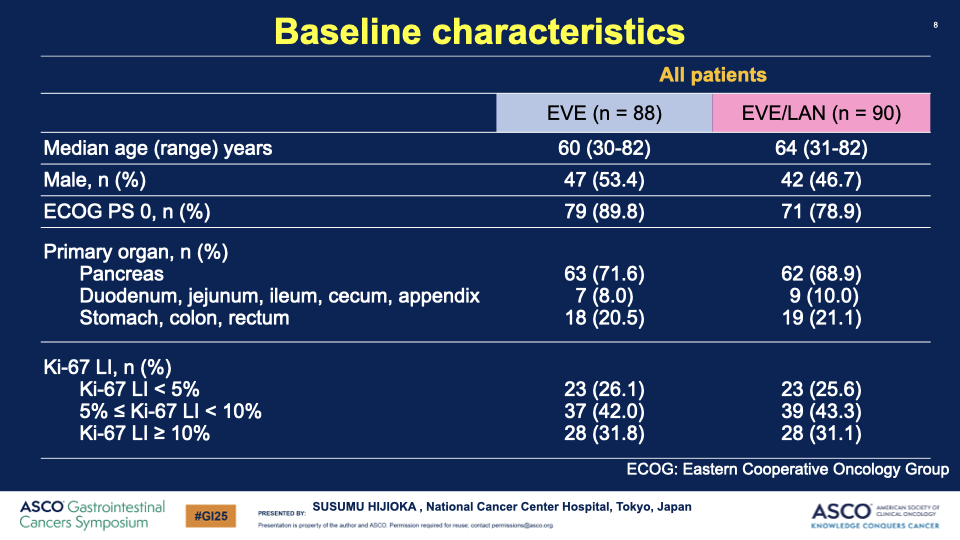
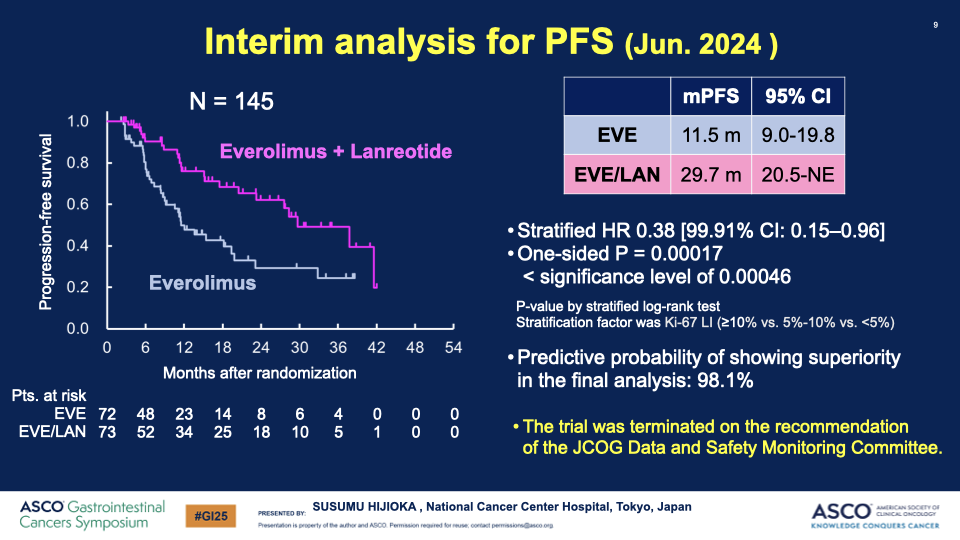
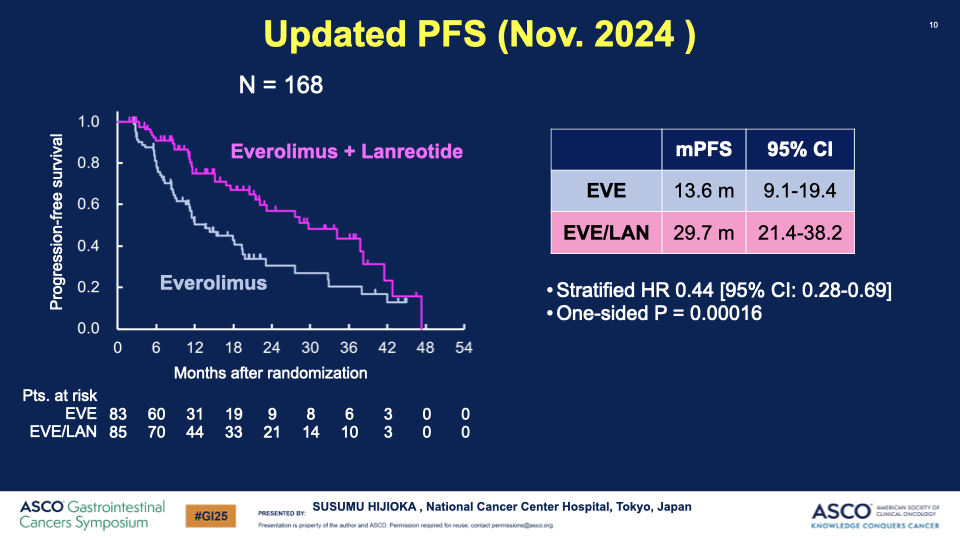

Additional Articles:
Resources:
