On June 3, 2025, the U.S. Food and Drug Administration (FDA) approved Darolutamide for de novo metastatic castration-sensitive prostate cancer (mCSPC), marking a notable advancement in prostate cancer care. This post delves into the data behind this Darolutamide FDA Approval 2025, its implications, and its significance for oncologists and researchers.
A 10-Year Evolution of FDA-Approved mCSPC Therapies
The progression of mCSPC treatment reflects a decade of FDA approvals, detailed in recent analyses. Key milestones include:
- 2015: Docetaxel – Introduced with a hazard ratio (HR) of 0.73, reducing the risk of progression or death by 27%.
- 2019: Apalutamide – Improved to an HR of 0.67, offering a 33% risk reduction.
- 2022: Darolutamide – Achieved an HR of 0.68, validated in the ARASENS trial with a 32% mortality risk reduction when combined with ADT and docetaxel.
- 2025: Darolutamide – Now approved for de novo mCSPC with an OS HR of 0.81, indicating continued survival benefits.
This evolution highlights Darolutamide’s expanded role without chemotherapy. Learn more about the FDA approval process for such therapies.
Efficacy Comparison Across Treatments
Efficacy varies across FDA-approved mCSPC therapies, as shown by hazard ratios. Darolutamide offers:
- Radiographic Progression-Free Survival (rPFS) HR: 0.54, indicating a 46% reduced risk compared to placebo.
- Overall Survival (OS) HR: 0.81, a 19% risk reduction in the latest data.
- Comparisons: Enzalutamide (OS HR 0.72, 28% reduction) and Apalutamide (OS HR 0.67, 33% reduction) show different profiles, influenced by trial populations.
These HR values, from trials like ARANOTE, emphasize the role of patient selection. The ARANOTE trial provides essential insights into Darolutamide with ADT alone.
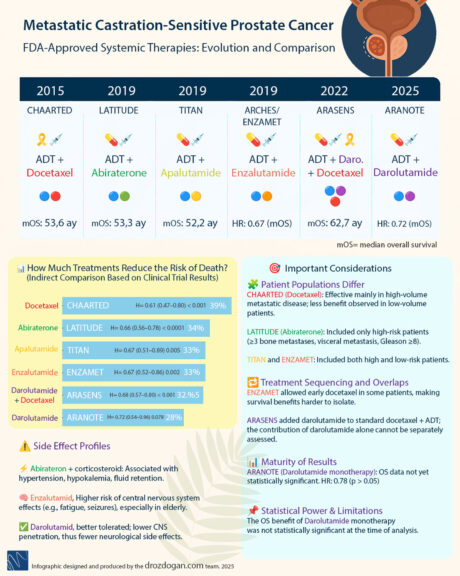
Image Courtesy: https://x.com/ozdogan_md
ARANOTE Trial Insights
The ARANOTE trial provides critical data on Darolutamide’s use in mCSPC:
- rPFS HR: 0.54, showing significant improvement over placebo.
- OS HR: 0.81, with immature data suggesting further follow-up is needed.
- Implications: Darolutamide with ADT alone may shift treatment protocols, but patient selection and safety concerns require further study.
This trial suggests Darolutamide could reduce chemotherapy reliance for eligible patients. Researchers can explore the full ARANOTE findings for deeper analysis.
What This Means for Oncologists and Researchers
The Darolutamide FDA Approval 2025 offers new opportunities for mCSPC management. Oncologists can tailor treatment plans using efficacy data and trial insights. Researchers should focus on:
- Patient Selection: Identifying ideal candidates for Darolutamide monotherapy.
- Safety Profiles: Assessing long-term effects compared to combination therapies.
- Future Trials: Exploring sequencing with other AR pathway inhibitors.
For more details, review the FDA’s official announcement.
Video Companion
Enhance your understanding with our video, “Darolutamide FDA Approval 2025: Prostate Cancer mCSPC Data.” It covers the 10-year timeline, efficacy comparisons, and ARANOTE insights in under a minute. Watch the video here (insert-video-link).
Conclusion
The Darolutamide FDA Approval 2025 for prostate cancer mCSPC represents an important step in treatment. With an OS HR of 0.81 and rPFS HR of 0.54, it provides promising options.
