Dive into the groundbreaking results of the EMBER-3 Phase 3 trial (NCT04975308) as presented by Dr. Virginia Kaklamani from UT Health San Antonio at the San Antonio Breast Cancer Symposium (SABCS). This trial focuses on imlunestrant, an innovative oral Selective Estrogen Receptor Degrader (SERD) specifically designed for patients with ER+, HER2- Advanced Breast Cancer (ABC) who have already been treated with endocrine therapy.
Key Findings from the EMBER-3 Trial:
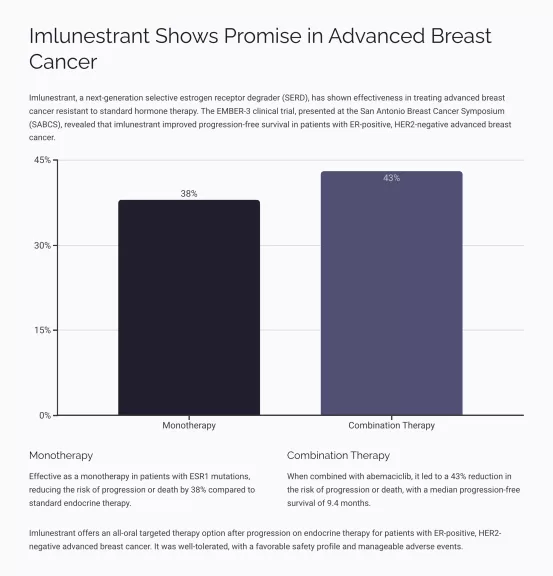
- Imlunestrant vs. Standard-of-Care: A dramatic leap in Progression-Free Survival (PFS) for patients with ESR1 mutations was observed, with a Hazard Ratio of 0.62 (P<0.001) and median PFS of 5.5 months compared to 3.8 months with standard care.
- Combination Therapy: When imlunestrant was combined with abemaciclib, there was a significant PFS benefit across all patient groups, evidenced by a Hazard Ratio of 0.57 (P<0.001) and a median PFS of 9.4 months versus 5.5 months.
- Objective Response Rates (ORR): Patients on imlunestrant alone had an ORR of 12%, whereas the combination with abemaciclib boosted this to 27%.
- Safety Profile: Imlunestrant was well tolerated, with common side effects being fatigue, diarrhea, and nausea, mostly of grade 1. The combination with abemaciclib saw higher rates of grade ≥3 adverse events, mainly neutropenia.
- CNS Protection: Post-hoc analysis suggested that imlunestrant might offer protective benefits against central nervous system progression compared to standard care.
What Does This Mean for Patients?
- New Treatment Option: Provides an all-oral regimen as an alternative for those who have not responded well to previous endocrine treatments.
- Extended Progression-Free Survival: Particularly beneficial for patients carrying ESR1 mutations, potentially offering more time before disease progression.
- Strategic Therapy Management: The efficacy of combination therapy opens new avenues for managing advanced ER+ breast cancer.
Watch our video for an in-depth discussion on these findings, what they could mean for the future of breast cancer treatment, and how they might translate into real-world patient care. We’ll also touch on ongoing research and the questions that still need answers.
Timestamps:
- 0:00 – Introduction to the EMBER-3 Trial at SABCS
- 0:06 – Overview of the trial’s three arms: Imlunestrant alone, endocrine therapy alone, and the combination of Imlunestrant with Abemaciclib
- 0:28 – Detailed discussion on the significant PFS benefits for patients with ESR1 mutations
- 0:41 – Comparing progression-free survival across all patients under the combination therapy
- 1:05 – Exploring the implications of these results for treatment approval and patient outcomes
Don’t forget to:
- Like this video if you found it informative!
- Subscribe for more updates on oncology research and treatments.
- Comment below with your thoughts or questions about imlunestrant or the EMBER-3 trial.
#EMBER3Trial #Imlunestrant #SERD #BreastCancer #ERpositive #HER2negative #AdvancedBreastCancer #OncologyResearch #CancerTreatment #Phase3Trial #ESR1Mutations #ProgressionFreeSurvival #Abemaciclib #OralTherapy #EndocrineTherapy #ClinicalTrialResults #CancerCare #OncologyUpdates
Related Articles:
https://www.clinicaltrials.gov/study/NCT04975308
