Updates in Lymphoma: Advancements in Treatment Strategies
Date: August 12, 2023
Presenter: Elizabeth Brem, MD (Clinical Associate Professor)
In the ever-evolving landscape of oncology, particularly in the field of lymphoma treatment, significant strides have been made to improve patient outcomes and quality of life. Dr. Elizabeth Brem, a prominent figure in the field, delivered a comprehensive presentation on the recent updates in lymphoma treatment during the recent event. Dr. Brem is a Clinical Associate Professor renowned for her expertise and contributions to the field.
Disclosures:
Dr. Elizabeth Brem is associated with several pharmaceutical and biotech companies, reflecting her active engagement in clinical research and collaboration. These collaborations include participation in Advisory Boards for BeiGene, ADC Therapeutics, and Astra Zeneca, Speakers Bureaus for BeiGene, Astra Zeneca, SeaGen, Incyte/Morphosys, GenMab/AbbVie, and Consultancy for Caribou Biosciences.
SWOG S1826: Advancing Treatment for Classic Hodgkin Lymphoma
One of the highlights of the presentation was the SWOG S1826 trial, a groundbreaking randomized study focused on improving the treatment of advanced stage Classic Hodgkin Lymphoma (cHL). The trial aims to compare the efficacy of two treatment regimens: Nivolumab (N)-AVD and Brentuximab Vedotin (BV)-AVD.
Dr. Brem elaborated on the rationale behind this study. Despite the advancements achieved with BV-AVD, relapses still remain common (7-20%). Furthermore, variations in chemotherapy regimens used for pediatric and adult patients, as well as geographic differences, have highlighted the need for personalized and effective treatments. Dr. Brem emphasized that BV-AVD comes with added toxicity compared to ABVD in adults, leading to challenges such as increased neuropathy and febrile neutropenia. With a significant number of adolescents and young adults receiving consolidative radiation therapy, the risk of late morbidity and mortality necessitates the development of more effective and tailored treatment options.
Incorporating PD-1 Blockade into Initial cHL Therapy
Dr. Brem discussed how incorporating PD-1 blockade into initial cHL therapy has shown promise in terms of both tolerability and effectiveness. Studies of frontline PD-1 blockade in cHL have demonstrated favorable outcomes, with Nivolumab-AVD (N-AVD) showing excellent progression-free survival (PFS) rates. Notably, 1L Nivolumab-AVD in advanced stage cHL exhibited a 9-month median PFS of 92%, while sequential Nivolumab-AVD in early stage cHL achieved a remarkable 3-year PFS rate of 98%.
S1826 Study Design and Interim Analysis Plan
Dr. Brem delved into the intricate design of the S1826 trial, outlining the eligibility criteria, patient stratification, and endpoints. The trial’s primary objective is to compare the progression-free survival (PFS) between N-AVD and BV-AVD treatment arms. The study is designed to accrue 940 eligible patients and utilizes a stratified log-rank test for PFS testing. Dr. Brem outlined the interim analysis plan, which includes several analysis time points and critical p-values for futility and efficacy, ensuring a robust evaluation of the treatment outcomes.
Safety and Efficacy Findings
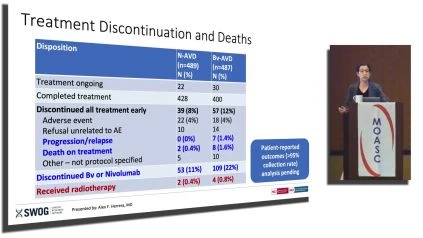
Dr. Brem provided an in-depth analysis of the safety and efficacy outcomes observed in the S1826 trial. Notably, N-AVD showed improvements in PFS compared to BV-AVD, with a 1-year PFS rate of 94% for N-AVD and 86% for BV-AVD. Furthermore, the presentation highlighted the incidence of adverse events (AEs) and toxicities associated with both treatment arms, including hematologic, infectious, and immune-related toxicities. Dr. Brem emphasized that N-AVD exhibited lower rates of certain toxicities, such as peripheral neuropathy, and reported a decreased need for growth factor support.
Conclusions and Future Directions
Dr. Brem concluded the presentation by summarizing the key findings of the S1826 trial. N-AVD not only improved PFS compared to BV-AVD but also demonstrated good tolerability and fewer discontinuations. The preliminary safety profile indicated lower rates of certain adverse events, particularly immune-related events. The ongoing follow-up will assess long-term safety, overall survival, and patient-reported outcomes, solidifying N-AVD’s position as a potential standard therapy for advanced stage cHL.
In closing, Dr. Brem posed the question: Is N-AVD the future for advanced stage Hodgkin Lymphoma treatment? The promising results from the S1826 trial suggest that N-AVD could indeed become a new standard therapy, paving the way for improved outcomes and enhanced quality of life for patients with advanced stage cHL. The presentation left the audience with a sense of optimism and anticipation for the future of lymphoma treatment.