Published on May 3, 2025
Antibody-drug conjugates (ADCs) have revolutionized oncology, offering targeted cancer treatments that minimize harm to healthy cells. Over the past 40 years, ADCs have evolved from experimental therapies to a cornerstone of precision medicine, with 11 FDA-approved ADCs and over 210 in clinical trials as of 2024. This article explores the history, mechanisms, challenges, and future of antibody-drug conjugates, drawing from insights published in Cancer Discovery.
What Are Antibody-Drug Conjugates?
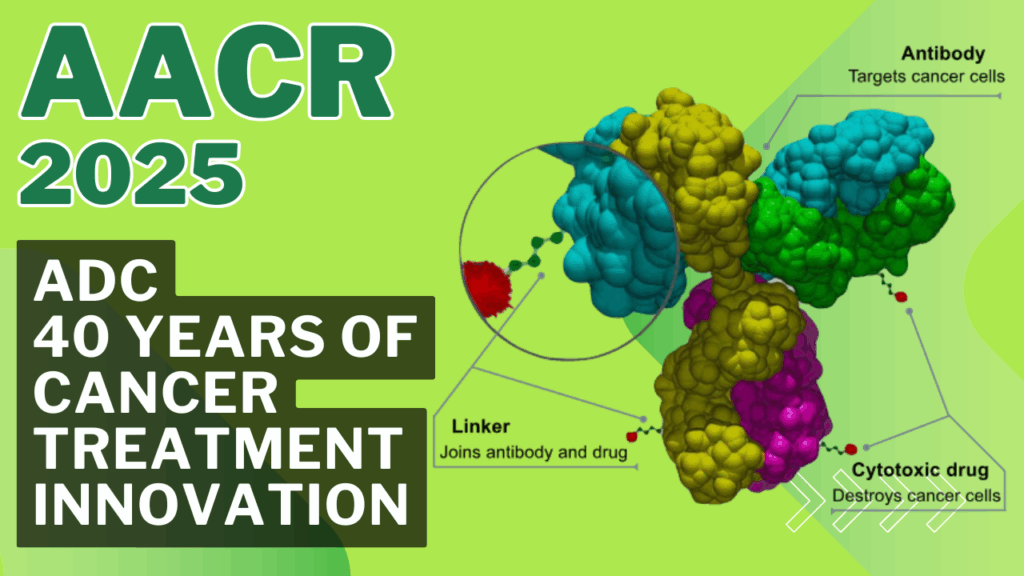
Antibody-drug conjugates are sophisticated therapeutics combining three key components:
- Monoclonal Antibody: Targets specific antigens on cancer cells, such as HER2 or CD33.
- Cytotoxic Payload: A potent drug, like maytansinoid or auristatin, that kills cancer cells.
- Chemical Linker: Connects the antibody and payload, ensuring stability in circulation and release inside the tumor.
This design allows antibody-drug conjugates to deliver chemotherapy directly to cancer cells, reducing systemic toxicity compared to traditional treatments.
The 40-Year Journey of ADCs
1980s: The Dawn of Antibody-Drug Conjugates
In the 1980s, researchers began exploring antibody-drug conjugates using murine antibodies. Early trials targeting leukemia antigens faced challenges like immunogenicity and unstable linkers, leading to high toxicity and low efficacy (response rates ~10-15%). These setbacks highlighted the need for better antibody design in ADCs.
1990s: Humanization and Refinement
The 1990s saw the shift to humanized antibodies for antibody-drug conjugates, reducing immune reactions. Trials like BR96-Doxorubicin achieved a 20% objective response rate (ORR) in solid tumors but struggled with off-target effects due to non-cleavable linkers. This decade emphasized linker chemistry optimization for ADCs.
2000s: First Approval and Lessons
In 2000, Mylotarg (gemtuzumab ozogamicin) became the first FDA-approved antibody-drug conjugate, targeting CD33 in acute myeloid leukemia (AML) with a 30% complete remission rate. Its withdrawal in 2010 due to hepatotoxicity (veno-occlusive disease in 5-10% of patients) underscored the need for safer payloads in ADCs.
2010s: Maturation and Expansion
The 2010s marked significant progress for antibody-drug conjugates with approvals of Adcetris (2011, CD30, Hodgkin lymphoma, 73% ORR) and Kadcyla (2013, HER2, breast cancer, 43% ORR). Cleavable linkers and potent payloads like DM1 improved efficacy. By 2019, four ADCs were approved, with trials exploring solid tumors.
2020s: Broad Applications
The 2020s have seen an ADC boom, with approvals like Enhertu (HER2, 61% ORR, 22-month PFS in breast cancer), Trodelvy (TROP2, triple-negative breast cancer), and Padcev (Nectin-4, urothelial cancer). Over 60% of trials now focus on breast (38%) and lung (22%) cancers for antibody-drug conjugates.
Mechanisms and Clinical Impact
Antibody-drug conjugates bind to tumor-specific antigens, internalize, and release their payload via linker cleavage. This targeted approach improves the therapeutic index, with studies showing 20-30% reductions in systemic toxicity compared to chemotherapy. For example, Enhertu’s bystander effect allows it to kill nearby cancer cells, enhancing efficacy in heterogeneous tumors.
Challenges in ADC Development
Despite successes, antibody-drug conjugates face challenges:
- Toxicity: Neutropenia (30% of patients) and interstitial lung disease (15% with Enhertu) remain concerns.
- Resistance: Mechanisms like antigen loss or efflux pumps reduce ADC efficacy in 20-40% of cases.
- Biomarker Identification: Only 70% of patients with HER2 or EGFR expression respond, necessitating better predictors.
The Future of Antibody-Drug Conjugates
The future of antibody-drug conjugates is promising, with ongoing research focusing on:
- Novel Payloads: Fifteen new payloads, like MMAF, are in trials, offering higher potency.
- Combination Therapies: Combining ADCs with PD-1 inhibitors increases response rates by 25% in early studies.
- Advanced Linkers: Site-specific conjugation improves stability, reducing off-target effects by 15%.
Conclusion
Antibody-drug conjugates have transformed cancer care, offering hope to patients with previously untreatable diseases. As research progresses, oncologists and researchers can expect ADCs to play an even larger role in precision oncology, with improved outcomes and reduced toxicity.
Learn more at aacrjournals.org.