Adil Daud, MD Professor of Medicine at The University of California, San Francisco, Director of the Melanoma Clinical Research, and lead author of the study SITCS 2020 abstract: Durable responses and immune activation with intratumoral electroporation of pIL-12 plus pembrolizumab in actively progressing anti-PD-1 refractory advanced melanoma: KEYNOTE 695 interim data.
Study
History The electroporated plasmid IL-12 (TAVO or telseplasmid tavokinogen) is a novel pro-inflammatory intratumor therapy with significant single-agent activity in melanoma that has been shown to synergize with anti-PD-1 antibodies in patients expected to be non-responsive to anti-PD-1.1 2 Interim results from patients with active progression of stage III/IV melanoma on anti-PD-1 antibodies was predicted.
Methodology
Patients with documented development of RECIST v1.1 disease have been enrolled after at least 12 weeks of treatment with pembrolizumab or nivolumab (or combination control point blockade) and within 12 weeks of the last dose (without intervening therapy). The number of prior therapy lines was not reduced. At least one usable lesion was electroporated every 6 weeks on days 1, 5, and 8 with plasmid IL-12 (pIL-12-EP), and pembrolizumab was administered every 3 weeks. RECIST v1.1 measured the tumor response in treated and untreated lesions every 12 weeks. ORR, secure, PFS, OS, and DOR provide endpoints.
Outcomes
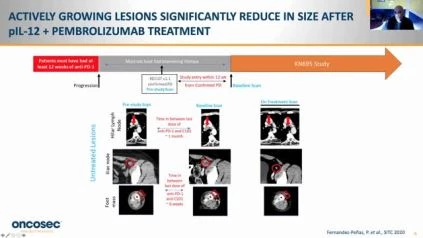
In this interim study, the first 56 patients treated out of 100 expected were included. Of these, 84% had stage IV disease, 30% had M1c or M1d disease, and 27% had previous ipilimumab use. The investigator-assessed ORR was 30 percent (3 CR/13 PR) in 54 efficacy assessable patients, 5 patients had 100 percent reduction of target lesions. All responses were confirmed, although only two responding patients advanced during the study 2 patients completed the study with continuing responses (figures 1 and 2). The ORR was 35.2 percent in patients with M1c/M1d disease (n=6/17). In 12 of 12 patients who had unaccessible lesions or accessible untreated lesions, tumor reduction was observed in untreated lesions. With a median follow-up period of 13 months, median overall survival (mOS) and length of response (mDOR) were not achieved. In 5.4 percent of patients, Grade 3 treatment-related adverse effects (TRAEs) were observed and there were no Grade 4/5 TRAEs. 23.2 percent was the incidence of grade 3 treatment-emergent (TEAEs) regardless of cause. The median time for treatment with pIL-12-EP was 10 minutes, and (range 2,46). Hallmarks of antigen-specific antitumor immunity were identified in this study, consistent with previous studies of single-agent pIL-12-EP, tumor IHC, and transcriptomic evaluations. Additional tests will be provided, including microbiome, TCR clonality, and assays of peripheral blood biomarkers.
Findings
The addition of pIL-12-EP to PD-1 antibody therapy induced a deep, stable, systemic response in local treated and distant visceral metastatic untreated lesions with nominal systemic toxicity in this rigorously specified PD-1 antibody refractory patient population.