Posted by Oncology Tube on February 5, 2026
Exciting developments are emerging in the treatment of myelofibrosis, particularly for patients with calreticulin (CALR) mutations. In this companion article to Dr. Angela Fleischman’s recent video interview and slide deck from the MOASC Hematology Spotlight (January 24, 2026), we explore preliminary Phase 1 data on the mutant calreticulin-specific monoclonal antibody INCA033989 — either as monotherapy or combined with ruxolitinib. Originally presented at the 67th ASH Annual Meeting in December 2025, these findings highlight potential disease-modifying effects beyond symptom control.
Whether you’re a hematologist, researcher, or someone affected by myeloproliferative neoplasms (MPNs), this post summarizes the key insights, clinical implications, and questions still to be answered.
Dr. Angela Fleischman, MD, PhD, hematologist-oncologist at UCI Health, sharing her perspective on emerging MPN therapies.
What Is Myelofibrosis and Why Do CALR Mutations Matter?
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) marked by bone marrow scarring (fibrosis), anemia, splenomegaly, debilitating symptoms, and increased morbidity/mortality. Mutations in exon 9 of calreticulin (mutCALR) occur in ~25–35% of MF cases. Higher variant allele frequency (VAF) correlates with more advanced disease, including worse anemia and elevated peripheral blasts.
Current therapies (e.g., JAK inhibitors like ruxolitinib) effectively reduce spleen size and symptoms but have limited ability to lower mutCALR VAF or target the underlying mutation.

Illustrations showing bone marrow fibrosis in primary myelofibrosis, with abnormal megakaryocytes and scarring disrupting normal blood cell production.
Key Findings from the ASH 2025 Presentation
The multinational Phase 1 studies (dose-escalation cohorts) evaluated INCA033989 in patients with myelofibrosis. The full abstract title captures the scope: “Safety and Efficacy of the Mutant Calreticulin-Specific Monoclonal Antibody INCA033989 as Monotherapy or in Combination With Ruxolitinib in Patients With Myelofibrosis: Preliminary Results From Dose Escalation of Two Global Phase 1 Studies.”
Dr. Fleischman’s slide deck emphasizes:
- No currently available mutant-specific treatments for CALR-mutated MF.
- INCA033989 shows promising reductions in platelet counts, mutant megakaryocytes, and CALR VAF.
- Combination with ruxolitinib may further improve spleen size reduction and symptom control.

ASH Annual Meeting & Exposition – Hematology.org
The vibrant atmosphere of the ASH Annual Meeting, where cutting-edge hematology research is presented.
Highlights from Dr. Fleischman’s MOASC Interview
In her January 2026 discussion, Dr. Fleischman expresses strong enthusiasm for the data:
- Impressive efficacy: “What’s so impressive is not only the reduction in platelet count, but even more so the reduction in mutant megakaryocytes in the bone marrow and the calreticulin variant allele frequency (VAF). The data really is much better than one would have ever anticipated.”
- Disease-modifying potential: Unlike JAK inhibitors, which don’t significantly impact mutant burden, INCA033989 demonstrates a “brisk reduction” in allelic burden — a rare and highly meaningful outcome in the MPN field.
- Combination benefits and cautions: Dual therapy may enhance spleen/symptom relief, but both agents can lower platelets, so “intense CBC monitoring” is essential to avoid significant thrombocytopenia.
- Key open questions: What happens upon discontinuation? Does mutant burden rebound, and how quickly? Impact on the mutant stem cell compartment? Durability of response? These will help determine if deeper responses — even potential “cures” — are possible.
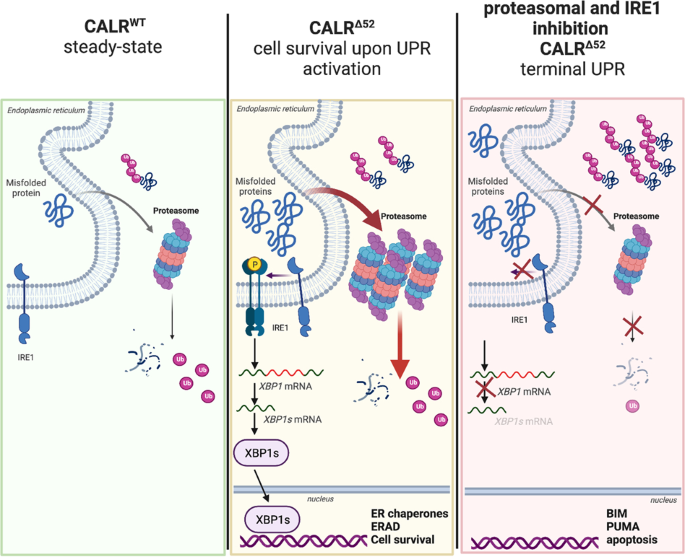
CALR-mutated cells are vulnerable to combined inhibition of …
Diagram illustrating cellular mechanisms involving mutant CALR in myeloproliferative neoplasms.

Splenomegaly Enlarged Spleen Stock Illustrations – 32 Splenomegaly …
Comparison of normal vs. enlarged spleen (splenomegaly), a common and burdensome feature of myelofibrosis.
Why This Matters for Patients and Clinicians
For mutCALR-positive MF patients, INCA033989 offers hope for more than symptom management — it targets the disease driver itself. Clinicians should watch for:
- Enhanced efficacy in combination regimens.
- Need for vigilant platelet monitoring.
- Future data on long-term durability and stem cell effects.
This could represent a paradigm shift in MPN therapy.
Access the Resources
- Full Slide Deck: Download the 52-page PDF from the MOASC presentation here.
- Video Interview: Watch Dr. Fleischman’s complete discussion here.
FAQs
What does INCA033989 target? It specifically binds mutant calreticulin, reducing mutant cell populations and allelic burden.
Is it safe with ruxolitinib? Preliminary data suggest tolerability, but combined platelet-lowering effects require careful monitoring.
When might we see more data? Longer follow-up, discontinuation studies, and stem cell analyses are critical next steps.
Final Thoughts
Dr. Fleischman’s insights underscore the optimism surrounding INCA033989 as a potential breakthrough for myelofibrosis. By addressing the root genetic driver, this antibody could change how we approach mutCALR-positive disease.
External Links:

