Featuring David Gandara, MD and Johannes Kratz, MD
Please visit www.oncocyte.com for more information on DetermaRx
Â
2020 has ushered in two important milestones in early-stage lung cancer. First, CMS approved DetermaRx, a molecular test that identifies patients with Stage I-IIA non-squamous NSCLC who are at high risk of recurrence, and may benefit from adjuvant chemotherapy. Second, the ADAURA trial results, presented at ASCO, demonstrated the efficacy of osimertinib in EGFR-positive early-stage NSCLC. In this webinar, we will review these latest research findings as well as ongoing clinical trials on the use of chemotherapy, targeted therapy, and immunotherapy in early-stage NSCLC. We will also present updated prospective data and clinical adoption trends for DetermaRx.
Â
Key summary points:
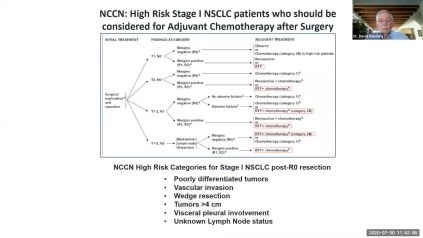
- Surgery alone does not cure 30-50% of patients with early-stage lung cancer; a better means of assessing recurrence risk is needed.
- Â The DetermaRx test has been validated in over 1600 patients to provide this enhanced risk discrimination and identify patients who are most likely to benefit from chemotherapy.
- DetermaRx is covered by Medicare and has experienced rapid clinical adoption in recent months with availability at 47 U.S. community and academic medical centers, and international availability in Israel, India, the Middle East and Africa.
- Clinical trials like ADAURA are ushering in additional adjuvant therapy options for early-stage non-small cell cancer