2023 ASH BCMA & Multiple Myeloma Updates with Slides
by: Lisa Lee, MD – Assistant Professor — Division of Hematology-Oncology, Department of Medicine, UCI School of Medicine
I wanna give you a quick update of the myeloma abstracts at ASH. And inevitably it’s gonna end up kind of focusing on the evolving saga over immunotherapies, because that’s the way that the field is heading. I have no conflicts of interest. So for those of you who treat myeloma, you know that we’re still plagued by this paradigm wherein we can’t tell patients that there’s a cure for their disease.
Each subsequent relapse herald’s a more shallow depth of response and a shorter time to progression. And also, I’ve witnessed firsthand the cumulative toxicities of their disease and prior treatments, which creates excessive burden on patients, especially close to the end. And this is what we’re working with.
How do we improve upon that? So there was a very interesting prospective study, a prospective observational study. It was called LocoMMotion. It was done in a multinational setting mostly Europe and the US with 248 patients. This observational perspective, observational study. Sought to recruit patients who fulfilled this criteria, which is that they were triple class exposed, had greater than or equal to three lines of therapy or double refractory to an imminent PI, and they were progressing on their last line of therapy.
So these patients were then observed as they were given standard of care therapy as their next line of treatment. And what this study found, is that the overall response rate for the next line of treatment hovered at around 29%. This graph shows you the progression-free survival curve on the left and the overall survival curve on the right, and we’re looking at a progression-free survival of 4.6 months and an overall survival of 12.4 months.
So this standard of care study provides the benchmark. The standard of care observational study provides the benchmark to which we can compare our studies of newer therapies in relapsed refractory multiple myeloma patients. So we’re very lucky. We have two approved cellular therapy products for patients. That represents the patient population as described in the previous study we have on the left, Idecabtagene vicleucel, otherwise known as Ide-cel.
The FDA-approved therapy overlaps with that of Ide-cel.
This was approved in March of 2021 for patients after they’ve received four lines of therapy and that they had to have been triple class exposed. On the right we have Ciltacabtagene autoleucel approved in February of 2022. Now last year. And the inclusion criteria for these patients to receive. The FDA-approved therapy overlaps with that of Ide-cel.
In the next few slides. I’d like to sum up the studies that led to these approvals, and again, that’ll give us the basis for comparison for future studies. So in this slide I have here listed for you on the left hand column the results of the KARMMA study, which led, which was a phase 1 B 2 trial, which led to the approval of Ide-cel on the right I have the result the characteristics of the patients who were enrolled on the CARTITUDE study, which was a phase 1 B 2 study, which led to the approval of Cilta-Cel. Now, the inclusion criteria was the same and it reflects, again, the standard. It exactly mirrors that standard of care study that I showed you previously.
Both studies excluded prior BCMA targeted therapy
Very important to note. Both studies excluded prior BCMA targeted therapy. The patient characteristic chart. Nothing of that was surprising. These patients were heavily pretreated. They were about six years from their prior from the time of diagnosis and had received a medium of six lines of therapy. And they were refractory, highly refractory group of patients.
So what about the efficacy outcomes? What I have here is I have the first column of all the patients who were in the KARMMA study. The second column are the patients who are, of these patients who were treated at the FDA approved dose of 450 times 10 to the million cell. And then the last column are the patients who received Cilta-Cel.
You can see that the overall response rates were 73, 81 and 97%. VGPR rates were approximately and I’m doing the math for you, 50%, 60%, and 90%. Duration of response hovered around 11 months for Ide-cel and they were not reached for Cilta-Cel after a medium follow up of 28 months, progression-free survival about a year, not reached, overall survival we’re coming up on two years. If you’re looking at this chart and you think, thinking, I was thinking that the numbers in the Cilta-Cel column look a little bit better. That’s actually true, there was a comparison study that looked at KARMMA compared to CARTITUDE and Cilta-Cel seems to have a edge over Ide-cel.
Now what I’m showing you in this slide is a comparison of patients with and without proposed high risk features in a representative BCMA CAR-T study. It’s actually the Ide-Cel study. And what this slide shows you is that the overall response rates for patients with high risk features were in general lower than those without high risk feature. And this translated into a decreased median progression-free survival for patients, for example, with extra medullary disease, high-risk cytogenetics, tumor cells burden this signal was not seen in patients who required bridging therapy or in patients who it was necessary to give more than one line of therapy per year. But it was deeply seen in patients who had revised ISS stage three compared with revised ISS stage one or two with a median progression-free survival of 4.9 months versus 11.3.
And what I’m trying to tell you here is not that I won’t use these products for these higher risk patients, but you have to explain to them the, your expectations for their outcomes. And you might also have to consider strategies on how to mitigate these these high risk features.
So for example one thing you can consider is it better to give car t cell therapy early on in the patient’s treatment course where for example, patients are less likely to develop, have extra medullary disease, and they are more likely to have lower tumor cells burden. So strategies like that have to be considered when you’re selecting patients for these real world BCMA CAR-Ts.
Can my patients get a BCMA CAR-T if they have already had a BCMA targeted therapy?
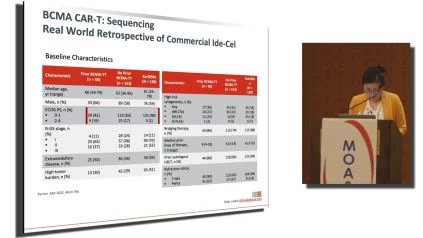
The other thing that I wanted to focus on was there was a, excellent oral presentation at ASH, which garnered a lot of attention. So this study addressed the question that many people have, which is can my patients get a BCMA CAR-T if they have already had a BCMA targeted CAR-T cell therapy? So what this study was a real world retrospective look at patients who got commercial Ide-Cel and compared the outcomes of patients who received prior BCMA targeted therapy compared to the patients who did not get prior BCMA targeted car t cell therapy. And compared to the patients who were on the original KARMMA study this is the baseline characteristic of patients and the there’s no surprise about the baseline characteristics. I only wanna point out that the patients who got Ide-cel the real world patients had a poor ECOG performance status compared to patients who are on the KARMMA study. That’s a given. , what I noticed was that patients who had prior BCMA therapy had more medium prior lines of therapy and had more penta refractory patients compared to patients who had no prior BCMA therapy.
And to me, this is comparing apples to oranges, but this is a retrospective study, so what can you do? So what kind of responses are we talking about? So if you give Ide-cel after what they observed is if you give Ide-cel after BCMA targeted therapy, you’re looking at overall response rate of 74% compared with 88% for patients with no prior BCMA therapy. Likewise, the depth is lower if you have received prior BCMA therapy. And indeed, both of these outcomes are statistically significant that the outcome for BCMA use prior BCMA use was inferior. But to be honest an overall response rate of 74% looks pretty good. Then the presenter went on to stratify the patients by the type of prior BCMA targeted therapy that they received. And what they showed here is that patients who received antibody drug con, probably compared to patients who had received a prior CAR-T cell therapy had worse outcomes. Yet again these overall response rates are pretty good. And what the presenter pointed out was that on this little chart on the right side, a patient’s response rate to their pre previous BCMA targeted therapy was incredibly low.
You can use BCMA CAR-T to salvage people who did not respond to their previous BCMA targeted therapy.
Low, okay? These numbers are very small, and this study is retrospective, but what this is telling me is that, You can use BCMA CAR-T to salvage people who did not respond to their previous BCMA targeted therapy. What about survival? They showed inferior survival with prior BCMA use. And then they showed that in a multi-variable, Very efficacy analysis that prior BCMA targeted therapy was associated with inferior best response pfs overall survival.
And this means that this, accounted for ac confounding factors of the fact that the prior BCMA patients had more lines of therapy and were more refractory. In terms of, and then they cut the data in a different way. What they showed was that in terms of, if you had to give BCMA by BCMA CAR-T cell therapy to a patient who had received previous BCMA targeted therapy the respond.
had a duration of their prior BCMA therapy that was less than the non-responders and the time to getting their CAR-T BCMA. CAR-T was longer compared to non-responders. Likewise, if you compared patients who received their prior BCMA targeted. more than six months ago compared to less than six months ago.
Patients who received their BCMA targeted therapy a while back had better overall response rates of 83 compared to 60% and better, greater than CR rates of 35 to 20. So my conclusion from reviewing this study is that you can treat. With standard of care, BCMA CAR-T. Even if they had received BCMA targeted therapies prior, the response rates were pretty good.
Although they were less than if they had never received BCMA targeted therapy. And then of these patients who had received a previous BCMA therapy, you wanna consider that patients who had shorter duration. BCMA targeted therapy a long time ago did better than patients who had longer duration and, maybe just progressing off of their B C M A targeted therapy.
So in addition to patient factors, there are many abstracts on optimizing the cellular product. Some themes that I saw shortening time to t-cell infusion. With the KARMMA and latitude studies, there are the 20% drop off from time patients harvested their autologous T-cells.
They’re getting the T-cell infusion. So ways to mitigate this, we can give patients allogeneicCAR-Ts off the shelf from other donors. There is also great interest for this manufacturing process that can make CAR-T-cells from autologous T-cells in less than two days. I thought that was really exciting.
Another theme is improving CAR-T expansion and persistence. The currently approved standard of. BCMA products have a mirroring CAR-T construct which generates potential anti BCMA antibodies taking the cars out of circulation while people are working on humanizedCAR-Ts to avoid this improving T-cell phenotype to be a earlier more memory like phenotype versus an exhausted.
an effectual T-cell phenotype is also being worked on. There was a presentation about dual targeting which showed incredible responses. I think the overall response rate for this product was like a hundred percent. And then of course, other targets are being looked at. So the field is really moving, so we have the standard of care on which.
Compare, we have the approved CAR-T-cells on to which to compare. We can only do better from there. Not to be outshined byCAR-Ts. We also have by specific T-cell engagers in multiple myeloma. We got our first approved by specific T-cell engager to Teclistamab, which is another anti BCMA bite.
The approval came in October. A few months ago, and this approval is for patients with the exact same indication as that for CAR-T cell therapy.
The field for BCMA targeted by specifics in myeloma is cro I wouldn’t say crowded is robust. I want to point out specifically elranatamab. This is likely going to be the next approved BCMA Bispecifics. We had this product at uci. At the phase two portion of the clinical trial as well as we have now the expanded access, and I find this product to be very easy to use and very efficacious.
The dose at which a rantin is given is a flat dose of 76 milligrams weekly as a subcutaneous administration. These patients were ego equally refractory to the other. Compared to the other studies that we have looked at the response rates that we expect is around 61% of patients. And we have all of the safety signals of the newer immunotherapies.
We have crs, neuro toxicities, infections, and neutropenia, but all at low and controllable.
So where is the where are the bispecifics headed? I think it’s headed towards first other targets. One of the most well attended oral abstracts at ASH this year was the monumental study and what the monumental one study. Was a multi-center open label, phase one two trial looking at take, which is a bispecific T-cell engager that recognizes GPRC5D in the monumental study.
The presenter Dr. Char from Mount Sinai. Presented on the phase two portion of the trial. In the phase two portion. They already have the recommended dose and they’re debating between ta given at 0.4 milligrams per kilograms as a subcutaneous drug every week versus Talquetamab given at twice the amount, but given every two weeks.
There was a third. A group of patients that were analyzed, which was a group of patients that had received prior T-cell redirection therapy, usually by specific T-cell engager or CAR-T-cell. And the primary endpoints was overall response rate as well as all of the usual response rates survival, MRD and safety, the baseline characteristics of this group of.
I’ve reviewed them for you. They’re the same as the previous studies. These patients, again, to highlight how refractory they are as shown here. So what kind of response rates are we looking at the overall response rates for both doses and schedules? Excluding the patients who had prior BCMA targeted therapy.
excluding patients who had prior T-cell targeting therapy was 70, about 74%. And the important thing to note that the, all the high risk subgroups experience similar overall response rates. So there’s no fallout with one of the high risk subgroups. This was a duration of response. Which showed a median duration of response of 9.3 months for all comers on the 0.4 milligrams per kilograms dose versus 13 months for the 0.8 milligrams per kilogram dose.
The patients who in Orange, who attained a CR of course, had better duration of response to this medication. The presenters pointed out that. that there’s significant AEs with this medication. The most notable AEs are hematological, ease and infections. It was interesting that he noted that the hematological AEs, namely the cytopenias actually got better as the patients progressed on their treat, on their treatment.
They saw a significant portion of crs about 79. and 72% icas were ex was experienced at 10%. Around 10%, but the higher grade CRS and icas were minimal. 35% of patients required tosi use and like we’re seeing with the bi BCMA by specifics 90% of CRS happened with step up dosing in the first full dose.
Something to know about. This target is that the G P R C five D is present on keratin producing cells. And so a special AE for this drug is skin nail and rASH due to the presence of this receptor on off target effects of this receptor. This last, I think it’s the last slide. This last slide is talking about patient’s response rates in that last.
That had prior T-cell redirection therapy. This is the patient. Characteristics. 70% of patients received a car. T 35 patients received a previous bispecific. The median duration of response I think is admirable. What’s really interesting to me is that if you look at this last row, the overall response by prior therapy patients who had bispecific as their prior therapy did a lot worse than patients who had a previous CAR-T.
So that was interesting to me, the overall response rate, still 62.7%. And so in conclusion, really excited. As a patient who treats as a doctor who treats myeloma, we have really good res options for late relapse. But I don’t see a cure yet. The slides, I, the studies that I showed you showed that the patient selection and strategic sequencing of therapies is.
Work is being done to optimize CAR-T cellular therapy and the product. I think that most patients will end up getting a BCMA bite, and I think that’s good. There’s a long wait for CAR-T therapy and not all patients may be eligible, and I’m glad to have that option. And in terms of sequencing I think that the.
the efficacy may be less for patients who received prior BCMA targeted therapy or patients who received T-cell a targeted therapy. But our newer therapeutics are able to salvage a significant portion of patients. So with that, I can conclude and answer any questions.
About the Author:
Hematologist and oncologist Dr. Lisa Lee practices at the University of California, Irvine Medical Center. She graduated from Rutgers Robert Wood Johnson Medical School with a medical degree before completing internal medicine residencies at Montefiore Medical Center and Tufts Medical Center, as well as a fellowship in hematology/oncology. She holds board certifications from the American Board of Internal Medicine in Hematology and Internal Medicine.