Zanubrutinib compared to ibrutinib in chronic lymphocytic leukemia
So I’m gonna start with chronic lymphocytic leukemia. And as part of late-breaking abstracts, we got this study which was zanubrutinib compared to ibrutinib before chronic lymphocytic leukemia.
Relatively straightforward study design. These were previously treated patients, so one prior systemic therapy, measurable disease, no prior BTK inhibitors, and they were randomized one to one to zanubrutinib or ibrutinib. The primary endpoint was progression-free survival and treatment continued until progression or toxicity.
And this was obviously a randomized study, so it was relatively balanced. The median age was about right for chronic lymphocytic leukemia in the late sixties. You can see here that the number of patients who had a 17 p deletion or an aberration and TP 53 was about the same in both groups at 23%. And then the majority of them in both arms were actually IGVH unmutated.
And the primary endpoint was pfs. And you can see there’s significant improvement in PFS for the zanubrutinib curve compared to ibrutinib. And in terms of looking at subgroups, if any particular subgroup benefited, you can see that more or less, everything favors the zanubrutinib. The things that kind of straddle one and have big error bars all are just relatively small numbers such as complex Caro type.
And when we look specifically at our higher-risk patients with 17 P deletion or TP 53 mutation, you can see there that the pfs is actually relatively similar to the cohort as a whole. In terms of adverse events. You can see that neutropenia was the most common on both arms for whatever reason. Maybe there were slightly more covid 19 related complications in the zanubrutinib arm.
But for the most part, they were relatively balanced. So I think this is the take-home in terms of reasons to choose one agent over another. You can see here that there’s a significant improvement in the number of cardiac events with zanubrutinib. In particular, I want you to focus on the serious cardiac adverse events where you can see that it was 7.7% in the ibrutinib arm and 1.9% in the zanubrutinib arm.
If you le look at the ones leading to treatment discontinuation, you can see that only one person discontinued zanubrutinib due to cardiac AE as opposed to 14 patients on ibrutinib. So this was a take-home point that was given during the late-breaking abstract, which was that zanubrutinib had a per impair superior pfs compared to ibrutinib.
The safety profile seemed improved, and this was, they called this the first study to Deen demonstrate PFS superiority in a head-to-head comparison. And we’re gonna talk a little bit more about that and what we do with that statement. So based on this last week, the FDA approves zanubrutinib for chronic lymphocytic leukemia.
So we already had a label in mantle cell lymphoma, Waldenstrom’s, and marginal zone. And so now we have the label in chronic lymphocytic leukemia as well. And I actually wrote my first script for on-label zanubrutinib this week. So this is the million dollar question. And I don’t know that there’s a clear answer to this because we don’t have a head-to-head study comparing zanubrutinib and acalabrutinib.
We just, zanubrutinib compared to ibrutinib and acalabrutinib compared to ibrutinib. And so this was the progression-free survival curve for Elevate rr. So this was a similar study to Alpine, in which it was patients who had one prior therapy for chronic lymphocytic leukemia, no prior BTK inhibitor, and they got randomized to either acalabrutinib or ibrutinib. And you can see here the pfs curves overlap now does that mean really that acalabrutinib is, or sorry that zanubrutinib is better because of their progression-free survival curse didn’t overlap. Is this a difference in populations? Who knows, right? This is why we’re not supposed to do cross-trial comparisons. If you look at the toxicity in Elevate, RR, I wanna also point out the atrial fibrillation here.
There was a fair amount of atrial fibrillation in the ibrutinib arm. Any grade was 16%, and grade three and above was about 4%. And there was still atrial fibrillation. The a acalabrutinib arm, it was less than the zanubrutinib. Or sorry, in the ibrutinib arm. This number is higher than what we’ve seen quoted in a number of the zanubrutinib studies.
But again, different populations, possibly slightly different eligibility criteria. And bad luck can just play into this too, right? Cause this is a population that’s not that uncommon to see atrial fibrillation anyway. And again, this is just to compare to what we’ve saw for zanubrutinib. Which again, which B T K inhibitor do we use?
Now, there was a slight trend towards overall survival benefit for acalabrutinib compared to ibrutinib. Now this is a secondary endpoint. The hazard ratio on this includes one, so we can’t actually say anything about it other than maybe there’s a suggestion of an overall survival benefit and maybe we’ll see it over time.
That was relatively similar to what was seen in Alpine, where again, big hazard ratio included one secondary endpoint, but a trend towards an improvement in overall survival for zanubrutinib. So both of them seem to have that trend. So how do you decide? If you look at the raw numbers, it does look like maybe zanubrutinib has less cardiac toxicity.
But again, there’s no direct comparison. This could all be from a slightly different patient population or eligibility criteria. So we can’t really make any conclusions there. And maybe as we look at both of these drugs over more time, we’ll see it. Maybe they’ll balance out In terms of things for the patient zanubrutinib, you can take it once a day or b i d, but it is a total of four capsules versus acalabrutinib, which is two capsules.
So the total number of pills someone is taking per day is lower with acalabrutinib
So the total number of pills someone is taking per day is lower with acalabrutinib. Some people really like to give the anti CD 20. It does get the white count down faster. And so you do have the option of giving the obinutuzumab with the acalabrutinib per elevate, tn, which you don’t necessarily have an approval of zanubrutinib with an anti CD 20.
The PPI issue is gone with both drugs. EALA changes their formulations so that there’s no longer a PPI problem. And I wonder if actually payers are gonna actually dictate some of this if, depending on how these drugs get priced, if certain payers are gonna have a preference. Obviously we don’t know zanubrutinib who just got approved last week, but that’s one thing.
I wonder if there’s going to be certain payers that push us to use one versus the other. I’m gonna switch gears for a minute to per ibrutinib, which is a non-covalent BTK inhibitor, which has gotten a lot of buzz over the last couple of years because it seems to show responses even when there is a progression after a covalent BTK inhibitor.
So this was an update from the Bruin study. And this is patients who previously had seen a covalent BTK inhibitor. And then we got treated with Iert ibrutinib, and you can see that there’s a really reasonable response rate. And here’s the waterfall plot. And you can see based on prior B T K I, although you can see the vast majority here was ibrutinib, but the handful of patients who got acalabrutinib or zanubrutinib also did.
People have been really impressed by the safety profile for per ibrutinib. You can see here that the number of grade three toxicities across the board are pretty low, except for neutropenia, which I think we’re just accepting is a class effective B T K inhibitors. . So what I didn’t put on the slide deck because it just happened on Thursday and I didn’t have a chance, is pert ibrutinib actually got an FDA approval in mantle cell lymphoma.
Many people are presuming at some point it’s gonna get a label in chronic lymphocytic leukemia probably within the next year or so. But right now we officially have this as an on-label indication in mantle cell lymphoma. And I wanna call your attention to one study that we have open with per ibrutinib. So this is for patients with previously treated chronic lymphocytic leukemia, so they need to have had a prior line of therapy.
there’s one cohort will, you’ll have seen a prior BTK and one cohort that won’t but you cannot have seen prior Venetoclax. So this is a randomization one-to-one of Venetoclax rituximab, which would be the on-label therapy versus veneta rituximab with per ibrutinib. So Kelly Kums, who recently joined us as the PI on this study, and feel free to reach out to her or me if you have any questions about it.
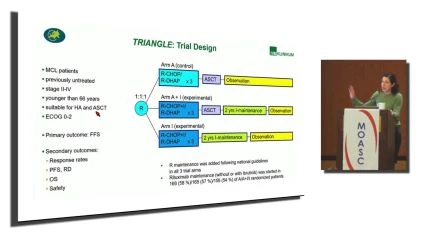
Now I’m gonna switch gears to mantle cell lymphoma. I think the, this was the most controversial abstract in the land of lymphoma. This was the triangle study. So this was a this was in the plenary session and this is basically questioning whether or not we need to be doing autologous transplantation in mantle cell lymphoma.
Watch and Share the Video Interview Here: https://oncologytube.com/v/41765
In the era of BTK inhibitors
In the era of BTK inhibitors. You can see the three arms here. So arm a. Kind of the standard of care. This came from Europe, so they used RCHOP alternating with D R D Hap followed by autologous transplant. And it’s not necessarily clear in the scheme of a patient’s didge, rituximab maintenance.
In the second arm there, they basically did the same thing. They just got ibrutinib. So the ibrutinib was part of the induction as well as the maintenance. And then arm eye there, or the third arm. Ibrutinib was added to the induction chemo. It was added to the maintenance, and there is no auto autologous transplant.
The response rate in all of the arms were exceedingly high. You can see there. For the kind of standard of care arm, it was 94% versus for both of the ibrutinib containing arms, it was 98%. So this is a free failure free survival. And they’re specifically comparing the. Middle arm, which was the chemo transplant and ibrutinib versus the standard of care.
So the top blue line there and all of these different things suggests an improvement of adding the ibrutinib to therapy. If you look at toxicity, you can imagine that adding the ibrutinib adds a little bit of toxicity, but it wasn’t all that bad, all things considered. You can see there that there were a little bit more cytopenias, maybe slightly more infections.
But both arm and there were slightly more in terms of grade five toxicities. But the numbers were overall very small. And so these were the conclusions that the authors. , which was that in terms of their primary endpoint, that the addition of the ibrutinib was superior to our standard of care.
I didn’t show you the curves here, but they felt like. The experimental arm where they got rid of the transplant, it was ibrutinib plus chemo and ibrutinib maintenance was, it wasn’t clear that the kind of standard of care with the transplant was better than this kind than avoiding the transplant.
And so they’re arguing or the arguer the person who presented this argue that maybe we are moving past autologous transplant in mantle cell lymphoma. I think it’s hard to say that. I think I still have a lot of questions about this because one thing that wasn’t at least presented here was r d, right?
Could it be that we didn’t need transplant because more patients got to an r d negative? Place because of the ibrutinib. And in general, we have seen that’s why high dose a C works, right? More patients become M R D negative, and so outcomes are better. So is it that maybe if we reach a level of M R D negativity, then we don’t need transplant and that’s why that ARM did so well.
The other question I have is, could you get away with less intensive chemotherapy?
The other question I have is, could you get away with less intensive chemotherapy? Alternating. D a p and RCHOP is rugged and could you get away? BR and a C or just br. Do you really need both of the therapies for maintenance? Do you really need ibrutinib and rituximab? Could you just use ibrutinib? Could you use a different BTK inhibitor?
And what about our transplant and eligible patients? What do we extrapolate from this? One qu, one answer we do have came from the Shine study last year, which is now published in the New England Journal, which was looking at br with or without ibrutinib. And certainly it’s another case arguing that.
Adding ibrutinib to chemotherapy works better than chemotherapy alone. My big question whenever I looked at this data was what about ibrutinib alone? Do we even need the chemo? But. We don’t have that data. But if you look at the toxicity again, you will see that there is more toxicity when you add the ibrutinib to the chemo.
There’s a fair amount of cardiac toxicity even in the placebo group in this particular population, cuz this was an older patient population. So in the intergroup, we’ve tried to look at this a little bit. So this was a study that is still enrolling, although to be frank, is probably gonna close relatively soon.
Looking at Arm A, which is b r. With Cytarabine. Then r b is BR with Cytarabine and acalabrutinib, and then RM C was just BR with acalabrutinib. So RMC is closed due to some pre-planned analysis and it didn’t show superiority to the other arms. Now whether or not that was a correct endpoint is a different story.
So that arm is closed. The other two are still enrolling, but I believe this is gonna close relatively soon. So at least it’ll give us a little bit more insight into these questions. And then the other study that’s ongoing in the intergroup, so though again is likely to close relatively soon, is really looking at this question of, do you need an autologous transplant if patients are M R d negative?
So what they’re doing here is patients can get whatever induction they want at the end of induction, they’re being checked for M R D. If you’re MRD negative, you get randomized to transplant or no transplant. And if you’re MRD positive, you go to. Plant and if your m r D can’t be detective, I believe it’s the adaptive assay, then you also go to transplant and everybody is getting maintenances maintenance, rituximab.
So maybe this’ll give us some insight into the question of, do you really need an auto autologous transplant for consolidation? No matter what you used as induction, if you’re m r d negative, The other study in the mantle cell realm that I just want to throw out there is we do have one for older patients who are not transplant eligible with newly diagnosed mantle cell lymphoma being randomized, one to one to zanubrutinib and rituximab versus br.
If you are interested, Dr. Pinner Brown’s the PI on this one. So I’m gonna focus for a minute on follicular lymphoma because we just got our first approval of a bispecific antibody in lymphoma within the last couple of weeks. So most inotuzumab, which is a CD 20, CD three bispecific antibody, recently got F D A approved for follicular lymphoma.
And just to give you some insight of efficacy, this was. I forgot exactly which patient population this was. I think this is looking at follicular lymphoma patients. And this was a long-term follow up and you can see here that the overall response rate was 78%. And then they had looked at the patient’s prior line of therapy where the response rate was about 55% and about 60% of these patients achieved a cr.
It does seem particularly for patients who achieve a cr, some of the responses are quite durable. , and this’ll be interesting to see how we’re all going to put it into clinical practice. So as compared Tolen Cito, right? Where we admit patients to the hospital as opposed to Tely, which has got approved in myeloma, where we do have to admit the patients for the first few days for the step up dosing, this one is approved to be given entirely outpatient.
So there’s the schedule for you. So we’ll all have to think about how we wanna do this practically in terms of. Follow up visits, have the patient call us if they have any evidence of crs exactly how we’re gonna put this into practice. But theoretically, if you wanted to give this, you could do it next week, completely outpatient in your office.
I haven’t done it yet. I’ll keep you posted on how it goes. And Bispecifics are coming for diffuse large B-cell relatively soon. None of them are actually approved yet, but I think we should be seeing probably the first approval for D L B C L this year. This was a study of patients who got GIB on compassionate use protocols, so this is going to be a sicker population than what’s going to go on a clinical trial.
But you can see there that there were still reasonable responses and some of them were relatively dur. So we do have a few studies for bispecific antibodies for diffuse large B-cell lymphoma. One is the Regeneron antibody, and I’m not gonna try to pronounce it begins with an O. Right now that this cohort is only available for after prior CAR T-cell therapy, but we’re expecting them to potentially open up other slots and indications as well over the next few.
For your third line and beyond diffuse large B-cell lymphoma patients, if one of the things you’re thinking about giving them is more chemotherapy, you could consider them for this, which is where the patients are getting randomized to an anti-bispecific antibody. In this case, the epco versus investigator’s choice of BR or R gemox.
And to be frank with you, it’ll be R gemox if you send them to me. . And we also just opened this one, in fact, just consented the first patient yesterday. This is adding the G gab to our ice. So this might be the patient who you’re thinking might still make sense to get salvage therapy and go to auto, or if you feel like you need something relatively intense to bridge them to CAR T-cell, for example, if you’re thinking about giving them ice anyway, consider sending them this way.
I do wanna talk for a minute about CAR T-cells
I do wanna talk for a minute about CAR T-cells cause I just wanna make it clear known that we do have them. We now have on-label kite products. So we have axi cell for D L B C L and follicular. We feist, I think our first patient this week and are gonna far our second patient next week. And that also includes brex cell for mantle cell lymph.
We have several products on study some of which are allogeneic targeting both CD 19 and CD 20. And if Dr. Lee didn’t pitch this, we do have a couple of options for multiple myeloma on study as well, targeting CD 38 and hopefully relatively soon, one for BC m a as well. And I wanna introduce you to a couple of my colleagues who couldn’t be here today.
Call Coombs, recently joined us. She trained at Sloane Kettering and has been at UNC for the last several years. She’s gonna be seeing the lion share of the chronic lymphocytic leukemia going forward, as well as some MDs and a few other related disorders. My call. Colleague Dr. Collette came to us from Mount Sinai, had trained at Columbia with some wonderful T-cell lymphoma experts, so he’ll be helping Dr.
Pinner Brown and I with the aggressive T-cell lymphomas and B-cell lymphomas. And with that, I will end and take any questions if we have time, like share and subscribe here on Oncology two. Begin notifications when similar videos are available.
About the author: Elizabeth Brem, MD
Elizabeth Brém, MD, is a hematologist-oncologist and medical oncologist at UCI Health who specializes in the diagnosis and treatment of lymphoma and other hematologic malignancies.
She received her magna cum laude in medicine from the University at Buffalo Jacobs School of Medicine and Biomedical Sciences in Buffalo, New York. She subsequently completed a residency in internal medicine and a fellowship in hematology-oncology at Beth Israel Deaconess Medical Center in Boston, where she was a clinical fellow at Harvard Medical School. In Boston, she also completed research training in the lab of Dr. Anthony Letai at the Dana-Farber Cancer Institute, focusing on the Bcl-2 family of proteins and its role as a therapeutic target and source of drug resistance in lymphomas.
Brém is a member of the American Society of Hematology, the Lymphoma Working Group of the Southwest Oncology Group, and the University of California Hematologic Malignancies Consortium. Her objective is to create and perform innovative clinical studies for lymphoma patients.
She treats patients at the Chao Family Comprehensive Cancer Center in Orange and at the UCI Health Cancer Center — Newport in Costa Mesa.