Inotuzumab, GMALL, Ponatinib & Blinatumomab ASH Updates in Acute Lymphoblastic Leukemia
By Van Huynh, MD
Updates in acute lymphoblastic leukemia that was presented at ASH in New Orleans. So I think there were so many great talks. There were so many great talks and I decided I would focus on just three and really go into the details of each of those talks. I think there’s a theme there. So the first will be The trial by the gm, o l, which is the outcome of patients with newly diagnosed pH negative ALL treated according to a pediatric based regimen.
Next I’ll discuss the use of immunotherapy in with Inotuzumab for patients older than 55 who are newly diagnosed in the GMALL initial one trial. And then I’ll end it with data from the Ponatinib Blinatumomab trial for patients newly diagnosed with pH positive ALL. And you’ll see that there are some encouraging results from this phase two.
So with regard to the first study, this was presented by Dr. Nicola Gupte, and she looked at the outcome of eighty a hundred and forty one patients who were eligible for evaluation who had newly diagnosed pH negative, acute lymphoblastic leukemia treated according to a pediatric based regimen in the GMALL protocol.
That’s the German multi-center. Adult, acute lymphoblastic leukemia trial and this I think had 142 sites. In this study, so as many of us know, so first of all in comparing pediatric to adults, ALL is first of all the most common cancer in pediatrics. It makes up the majority and then after that we have lymphoma brain tumors.
Unlike adults pediatrics with acute lymphoblastic leukemia do amazing, they actually have an overall survival of at least 90 to 95%.
But I think because it’s so common, we know a lot about it. . Unlike adults pediatrics with acute lymphoblastic leukemia do amazing, they actually have an overall survival of at least 90 to 95%. There is a subgroup, what we call low risk. Their overall survival is actually 98.6. So it’s pretty phenomenal. So going back to this, when you look at they presented the registry Swedish registry, and as you can see the overall survival is pretty.
Is there a pointer? Here?
Is pretty poor. Especially for the older patients here that were 75 to 84. The younger patients less than 30 had a much better overall survival. And when they look at data from 2005 to 2012 for patients, I’m sorry, over the age of 55 So about 79% were offered induction chemo and 21% palliative.
Even those who were offered induction chemotherapy, their overall survival was at three years, was only 33%. So it’s pretty dismal. And if, of course the patient went on to palliative care, it was 3%. And that’s just some background. When you use immunotherapy. That’s something that’s being used more in the adult world for acute lymphoblastic leukemia.
This is some abstracts that were presented in 2021, showing that Inotuzumab, along with Len Nitum actually offered pretty favorable outcomes. When you look at this study, they looked at the registry going back as late as 2003. And these were patients over the age of 65. They started to the study way back at that time and they were using a pediatric based protocol at this time.
They didn’t use asparaginase during induction, using it more in consolidation as the study evolved. And here are some other salient points here. I won’t go into details, but I’ll go right into what this presentation really covered was that they broke patients up into two groups. And again, this was over a span of nine 18 years, a span of 18 years, and they broke this up into two groups.
You have group one and you have group two. Group one really evolved over the years, and this is just the difference that you see in how it changed because Group one had different cohorts. I won’t focus on that, but really the focus is to take aways is group two. And what was done different is that they did use asparaginase, but instead of using it during induction chemotherapy, it was actually.
Delayed until consolidation. The other thing is that they started to use more MRD based evaluation to look for a response. And based on that, the patients would be risk stratified. And also decisions regarding transplant were made using minimal residual disease. So that was group two. The characteristics.
In this study there were 741 evaluable patients. The medium age was 68. If you look at here there were about 11% who older than 75, who were offered therapy and to be a part of this study. 39% were 50, 60, 65, and the rest were somewhere in between. The majority had B lineage 80%.
I wanted to focus on this. 76% had a white count less than 30, and 24 was less than was greater than 30,000. And here you, they also looked at the BMI and whether obesity had an impact on their survival. This is just more detail in the, some of the subtypes won’t go into that. Majority of the patients had a good Charleston score of less than two.
So how did these patients do? So they looked at the response rate in acute lymphoblastic leukemia at the 841 patients.
And were fit to be on the study. And again, 142 centers. . So how did these patients do? So they looked at the response rate in acute lymphoblastic leukemia at the 841 patients. The CR rate, meaning the the incidence of being an M one marrow, less than more morphologic remission of less than 5% was 73% in both groups, one and two.
And the , the early deaths was 14%, and the failure rate was 13%, meaning they were still an M three at the end of at the end of induction. So then you break this up into group one and group two. And remember group two is a group where they did asparaginase but saved it for consolidation thereafter, and using Mr.
D. Group one definitely had more patients, 593. Group two was 248. You saw that the CR rate was the same, right? 72 and 75. So really no difference. However, I think what was striking was that the incidence of early deaths was 15% in group one, and 9% in group two. . And then this sort of just breaks it down further.
In terms of the CR and early deaths, I think what’s remarkable or striking is that age, I think we would, it makes sense, does make a difference. Those who are younger, 56 to 65 had a CR rate of 76 compared to 63. For those. Older than 75. And same with the early deaths. Huge difference. 9% for the younger group versus a quarter, 26% for the older groups.
Going down, I think I wanted you to take a note at the white count at this is a peripheral blood cell white count at initial induction for those who are at less than 30, 30,000 wbc. Their CR rate was 77 compared to 62 with those with a higher white count. And I think there was maybe a little bit of difference.
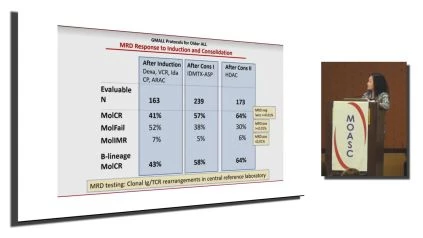
Those who were obese didn’t fare as well as patients who were less not obese or overweight. So what was the minimal residual disease response at the end of induction and also at the end of consolidation. . So at the end, after the first induction, there was 163 patients who were valuable. Those who had a molecular cr, meaning that they cleared their r d and r d was tested using clonal, immunoglobulin and T-cell receptor rearrangements.
The definition of negative minimal residual disease means that your MRD is less than 0.01, and so the percentage of those who achieve that molecular CR are or MRD less than 0.01. Was 41% after induction, 1 57 after consolidation after the first consolidation, and 64 after the second consolidation.
And so what about the remission duration for this group of patients? So they first clumped it into both group one and group two, and the medium follow up was about over two and a half years at. The probability that a patient would be in remission at three years was 45% and at five years was 38%.
And then they broke it down further into group two and group one. And what they found was that if you were in group two, they had a higher probability being remission 62% at three years versus 41%. For those who were in group one, the earlier group.
So what’s the the impact of minimal residual disease response on their overall survival after consolidation? Two. So what this shows is that those at three years, if you had if you had a molecular cr complete remission after consolidation, two, your overall survival is gonna be higher at 80. . And so I think, and this pretty much says the same thing and it breaks it down to B lineage here, but really is that potentially, could we use earlier MRD based evaluation to make decisions and determine whether you risk stratify, upstage, downstage, or even take the patients to transplant.
So that was the purpose of this. And then what about patients who went on to get stem cell transplant? There were 51 patients who had who proceeded to stem cell transplant after first remission. Their average age meet an age was 64. And 14% had T-cell, 80% had B-cell. And the, and some were PEL.
So in this whole group of 51 patients, their overall survival at three years was 56%. And again, when you break it down by age, that those who are 56 to 65 fared much better with the 66% overall survival versus the older patients greater than 66 years old. Had a 41% at three years and then just breaking it down to T and and B-cell.
So the overall survival of this 51 T-cell patients was 59% at three years at, that’s, I think that’s pretty good. The average age of those patients was 63. , they then broke it down to B-cell patients. And this again was for group two, which I found interesting was that the B-cell had a three year overall survival less than the T-cell.
This is typically opposite for our pediatrics. And when you break it down in this group, two by age not surprisingly, the ones who were younger, 56, 65, did much better than the older patients. So the summary the authors concluded was that using this age adapted pediatric based regimen, there were reasonable complete response rates that could be achieved in patients up to 75 years.
But definitely when beyond that, their overall survival was pretty poor. The molecular CR rates meaning MRD less than 0.01, was lower in this group. Than in the younger patients. And that more intensive consolidation using Pegas barges could improve outcome to 62% in the youngest cohort in this group too, they did note that which I think makes sense is that in the older patients, or those with multiple comorbidities, really consider alternative protocols or immunotherapy or use a step rise reduction of chemotherapy.
And that was the point of showing you the side with immunotherapy at the beginning. So I think that all patients who are diagnosed with acute lymphoblastic leukemia should be referred to a specialized center and offered a really thorough workup and designed management protocols and opportunity to be on a clinical trial.
The next study I’ll move on to is the study presented by Mathos TALs from Germany. It is really an an offshoot of the previous study and it looked at inotuzumab induction followed by standard chemotherapy and that it could yield high remission rates in de novo B-cell, ALL in patients greater than 55 years of.
Watch and Share the Interview Here: https://oncologytube.com/v/41752
You probably know this, but maybe this could be for the fellows. So what is Inotuzumab? Inotuzumab is a monoclonal antibodies. It is conjugated to and it’s it’s a CD 22 monoclonal antibodies and it is conjugated to Calicheamicin is a very toxic Antibiotic. And so after it, the CD binding to CD 22 surface antigen on the leukemia, the inotuzumab were intern internalized into leukemia cell, then gets released and that’s when it binds to the DNA of the leukemia and just causes DNA breakage and ace cell apoptosis.
It’s generally given on a weekly dosing. Usually three three doses given weekly, and multiple studies have shown in the adult population that it’s been effective for the relapse population. And this, and I’ll just highlight a few, which is really the backbone of led to this study is that in, you probably already know this, but 70%, there was a 70% CR and c r I rate when it was used for relapse refractory patients, even those really with high disease burden.
And then in another study using inotuzumab in combination with low intensity, a mini hyper C V D. as a frontline for patients who were older, pH negative ll. They found that there was a low early death rate of just zero and that there was promising overall survival, 63% even at the three year mark.
And then the next study pretty much shows something similar, which is this is a promising treatment for patients. And the rationale thus led to this agent being used. In these pH negative patients. And so what they offered is that these patients got three cycles of inotuzumab and then there it was followed by conventional consolidation, and the patients were 55 years of age or older.
The primary objective was to evaluate the efficacy of IO in induction therapy and really look at the number of patients. Still alive in first remission after the first year. Secondary objective was to look at the safety profile and of this agent. . So to summarize, so what they did was they offered these patients three rounds of induction, of inotuzumab in the first cycle.
They used the dosage of 0.8, the first week, 0.5 second and third week. And it was of course used with dexamethasone. The M r D was assessed at the end of each induction cycle. , if the patients had molecular remission after the third cycle, then they were offered additional conventional therapy with six additional cycles of chemo that I’ve listed here.
And patients also had the option to proceed after that. Six cycles of therap chemotherapy with maintenance, with just oral methotrexate and six m. . So the primary endpoint really was to look at the event-free survival at the first year, and that was defined as having any persisting bone marrow blast after two cycles or relapse or death.
The secondary endpoint was looking at the remission rate, the rate of negative M r d the relapse-free survival, the overall survival, and the number of deaths. The hypothesis of this study was that If they were able to achieve an event rate, which means patients with without persisting bone marrow disease or relapse or death, and that rate was less than or equal to 40% at that one year mark, they would say that the study was successful enough that to do additional experimental treatment and do additional study.
And so that was really one of their, one of their goals. This study had 42 patients that were valuable. To be included. They were older than 55 years of age. They had CD two positive leukemia. Cuz again, that’s what Inotuzumab targets. They they could have c n s disease and they could not have pH positive, ALL or Burkitts, et cetera.
This is list shows the patient characteristics of the 43 patients. That their average age was about 64 years of age. Equal ratio of male to female. The majority had B-cell, acute lymphoblastic leukemia, and the CD 22 expression the medium CD 22 expression in their bone marrow was 69.
So what was the response rate? I think it was really remarkable cuz it showed that all a hundred patients had achieved CR or cr i after two rounds of induction. There were no early deaths and the number of patients who had MRD negativity after three rounds of induction was actually amazing. 74%. So it showed that this was , great agent as a single agent.
I’m gonna skip that. So what was the overall survival event-Free survival at one year. You have 91%, two years, 81%. EFS was really great as well. 88 and 73 at one and two years perspectively. And again, no patients die within the first six month and this was a pretty long, medium follow up of almost 700 days.
Okay, so what are the side. So the adverse effects there were no grade, there were no grade four or higher. Mainly you saw marrow suppression with leukopenia, nema, thrombocytopenia, some transaminitis, elevated bili. There was only one patient had v o d, which is always a concern with this agent.
So the conclusion was that this, I know, was effective as a monotherapy and first line. For newly diagnosed patient and that the toxicity was fairly low and tolerable. There was promising overall survival of 81% after two years. And I have five minutes or less. I’m gonna go onto the next one.
So the next study was presented by Nicholas Short from MD Anderson, and he looked at now ubma, another immunotherapy in combination. Ponatinib, a third generation TKI for patients who are pH positive acute lymphoblastic leukemia I’m not gonna go cuz you guys know Howum works. . And so a little bit of background is that most patients who are pH positive in the adult world, the standard of care is they many proceed to receive a bone marrow transplant, but even with that, their survival is low.
Secondly, a lot of patients have resistance and that’s the mechanism for relapse is that the mutations in the T3 15 i leads to relapsed. Often Ponatinib is a third generation tki and it’s been effective against this this Mutation. And so there’s been therapies with Ponatinib hyper C V D that’s been promising.
And then again, Lin Nitum, you all know, has been effective as well in in ALL patients. So the primary endpoint was to assess the complete molecular response rate. And then these are the secondary end. Inclusion criteria. This is actually a phase two study. They looked at patients, not only pH positive a l, but also the CML the a l, but also CML patients and also the relapse population.
This will focus only on the newly diagnosed pH positive a l. So here’s the overall schema of this study. I’ll break it down for you. So basically these patients underwent an induction where they received 30 milligrams of Ponatinib and four weeks ofatumumab continuously. That was the first month.
MRD was assessed. They then proceeded to four cycles of consolidation cycles, two to five, getting a lower dose of Ponatinib 15 milligrams. And with each cycle, they also continued their Blum. They then were offered a maintenance phase where they actually got ponatinib for a long time, 15 milligrams for five years.
There were 15 LPs or 12 LPs that were interspersed throughout their entire therapy. So what was the characteristics of these patients? There were 40 patients. Average age was 57. Let’s see. Okay what’s the response rate? It actually was was pretty excellent. Overall the com complete molecular response rate was 87%, so that was pretty remarkable.
And the complete remission was also pretty good at 96%. So serum monitoring of the peripheral blood bcr, A B L was done. And you can see at the beginning it’s a hundred percent very high, but there you could see a rapid reduction by the second week of the PR transcripts where 58% had complete remission in the peripheral blood.
And it got lower as this is cycle one, day 43 as they proceeded along the cycle. Out of those 40 patients, there’s one death due to post-procedure bleeding. There was an early death from intracranial hemorrhage. There are two patients that relapsed. One went on to get a transplant, and then the other 35 have an ongoing response and they have not needed a transplant.
Okay, so outcome is great. 92%. And here you. And event-free survival was pretty excellent. It was fairly well tolerated with. The typical side effects that you would see. This is just the side effects from Ponatinib. And then the next is some mild CRS and neurotoxicity that you see from the Lin Nitum.
So in conclusion, ponatinib and Lyna is safe and effective. You saw some deep and rapid responses and in add. The responses were durable and that it’s a promising combination therapy that can be transplant sparing for this high risk population. Thank you
About the Author: Van T Huynh, MD
Oncology CHOC Children’s Specialist Dr. Van Huynh. She holds board certifications in pediatric hematology-oncology and general pediatrics. Her clinical interests include immunotherapy, bone marrow transplant, juvenile acute lymphoblastic leukemia (ALL), and relapsed leukemia.
Dr. Huynh studied medicine at the California’s UC Irvine College of Medicine. She served her fellowship in pediatric hematology-oncology at CHOC Children’s Hospital after completing her pediatric residency training at Harbor-UCLA Medical Center. She serves as the primary investigator (PI) for the Therapeutic Advances in Childhood Leukemia/Lymphoma (TACL) Consortium, a multi-center partnership that provides patients with relapsed or resistant leukemia and lymphoma with novel therapeutic choices. At CHOC Children’s, Dr. Huynh is the PI for the ZUMA-4 CAR T-cell Trial and is in charge of the CAR T cell program.
Clinical Affiliations
Allergy immunotherapy, bone marrow transplant, relapsed leukemia, and childhood acute lymphoblastic leukemia (ALL)
At Orange’s CHOC Hospital, Dr. Van T Huynh works as a staff member.